Using Conductivity to Find an Equivalence Point
Experiment #26 from Chemistry with Vernier
- Education Level
- High School
- Subject
- Chemistry
Introduction
“
In this experiment, you will monitor conductivity during the reaction between sulfuric acid, H2SO4, and barium hydroxide, Ba(OH)2, in order to determine the equivalence point. From this information, you can find the concentration of the Ba(OH)2 solution. You will also see the effect of ions, precipitates, and water on conductivity. The equation for the reaction in this experiment is:
Before reacting, Ba(OH)2 and H2SO4 are almost completely dissociated into their respective ions. Neither of the reaction products, however, is significantly dissociated. Barium sulfate is a precipitate and water is predominantly molecular.
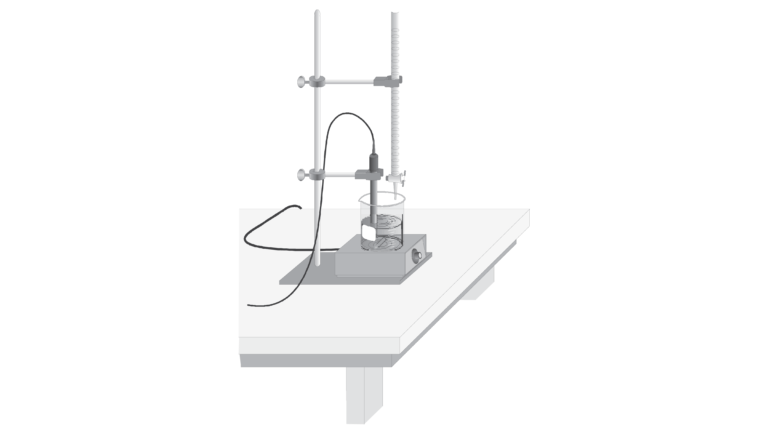
As 0.02 M H2SO4 is slowly added to Ba(OH)2 of unknown concentration, changes in the conductivity of the solution will be monitored using a Conductivity Probe. When the probe is placed in a solution that contains ions, and thus has the ability to conduct electricity, an electrical circuit is completed across the electrodes that are located on either side of the hole near the bottom of the probe body. This results in a conductivity value that can be read by the interface. The unit of conductivity used in this experiment is microsiemens per centimeter, or μS/cm.
Prior to doing the experiment, it is very important for you to hypothesize about the conductivity of the solution at various stages during the reaction. Would you expect the conductivity reading to be high or low, and increasing or decreasing, in each of these situations?
- When the Conductivity Probe is placed in Ba(OH)2, prior to the addition of H2SO4.
- As H2SO4 is slowly added, producing BaSO4 and H2O.
- When the moles of H2SO4 added equal the moles of BaSO4 originally present.
- As excess H2SO4 is added beyond the equivalence point.
“
Objectives
In this experiment, you will
- Hypothesize about the conductivity of a solution of sulfuric acid and barium hydroxide at various stages during the reaction.
- Use a Conductivity Probe to monitor conductivity during the reaction.
- See the effect of ions, precipitates, and water on conductivity.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #26 of Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.





