An Oxidation Reduction Potential (ORP) Sensor measures the activity of oxidizers and reducers in an aqueous solution. It is a potentiometric measurement from a two-electrode system similar to a pH sensor. Sometimes it is also referred to as a redox measurement. Unlike a pH sensor, an ORP sensor measures the ratio of oxidized to reduced forms of all chemical species in solution.
The ORP sensor is made up of two electrochemical half cells where the reference electrode is generally Ag/AgCl and the measurement electrode is commonly Pt. The potential difference between the two electrodes represents the redox potential of the solution being measured and can be described by the Nernst equation.
E = Eo – 2.3 (RT/nF) x (log [Ox] / [Red])
Where:
- E = total potential developed between the measurement and reference electrodes
- Eo = a voltage specific to the system
- R = gas constant
- T = temperature in K
- n = the number of electrons involved in the equilibrium between the oxidized and reduced species
- F = Faraday constant
- [Ox] = concentration of the oxidized species
- [Red] = concentration of the reduced form of that species
The output of the ORP sensor is relative to the reference electrode. For example, a reading of +100 mV indicates the potential is 100 mV higher than the potential of the reference half cell and suggests an oxidizing environment. Likewise, a –100 mV reading indicates a potential 100 mV lower than the reference half cell and is a reducing environment. In some applications, redox potential may be reported as Eh which is the voltage reading with respect to the Standard Hydrogen Electrode (SHE). By taking into account the offset of the reference electrode used in the ORP sensor, the potential can be converted into Eh readings. Vernier ORP sensors use a Ag/AgCl saturated KCl reference electrode.
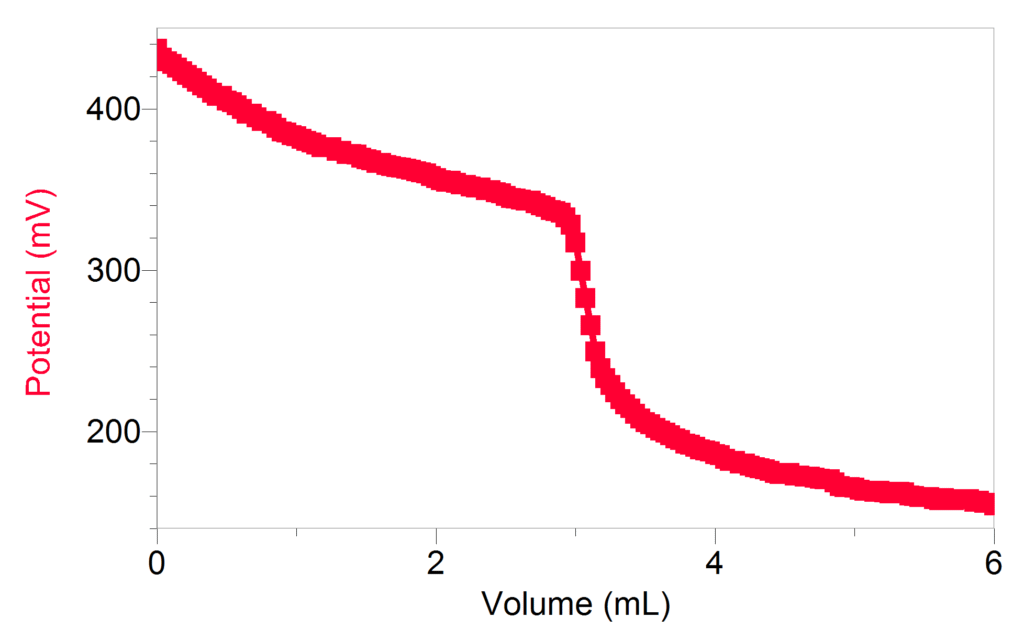
In education, a common application for an ORP sensor is a potentiometric titration. Similar to an acid-base titration, a titrant is added to a sample incrementally until all the sample has reacted and the end-point is reached. One example where students can apply their understanding of redox is by using a Vernier Go Direct ORP Sensor to determine the concentration of H2O2 by titrating the solution with KMnO4. To correctly calculate the concentration, students must understand the balanced redox reaction between KMnO4 and H2O2.
Vernier Tip: Check out two additional experiments from Vernier using an ORP Sensor.
