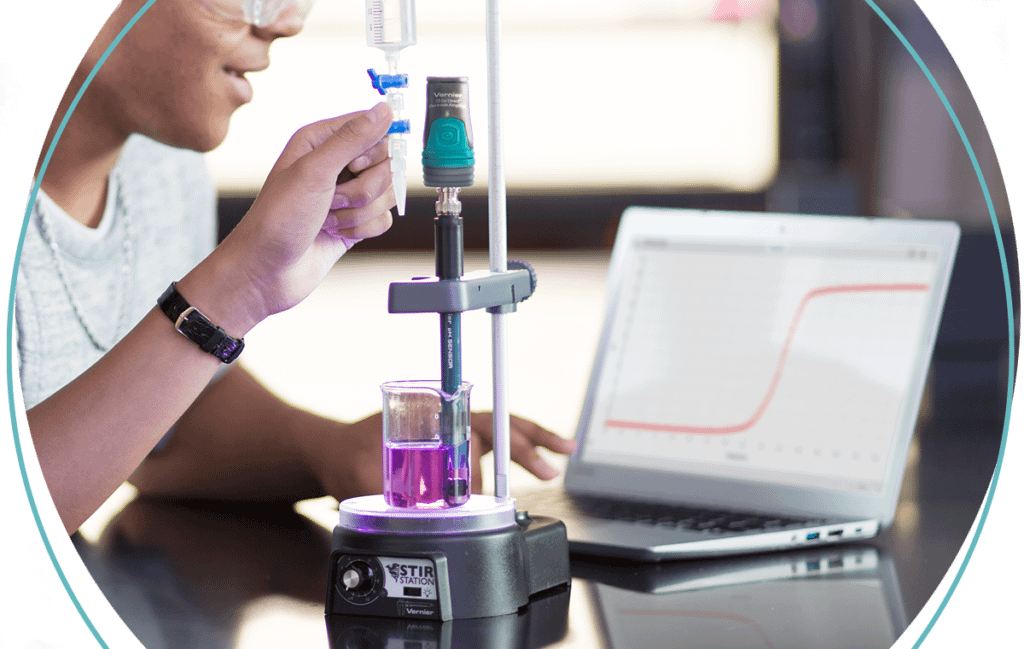
Determining the Percent Peroxide in a Commercial Product
Hydrogen peroxide, H2O2, is notoriously unstable and light sensitive, and therefore it is difficult to maintain a solution of H2O2 at a specified concentration. Commercial hydrogen peroxide is packaged in an opaque bottle, usually a brown color, to shield the H2O2 molecules from light. The concentration of commercial hydrogen peroxide is usually 3% (w/v).