Heat of Combustion: Magnesium
Experiment #19 from Chemistry with Vernier
- Education Level
- High School
- Subject
- Chemistry
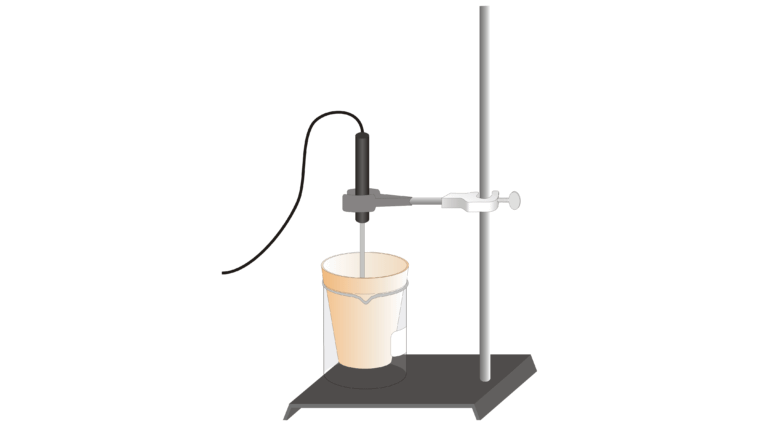
Introduction
In Experiment 18, you learned about the additivity of reaction heats as you confirmed Hess’s law. In this experiment, you will use this principle as you determine a heat of reaction that would be difficult to obtain by direct measurement—the heat of combustion of magnesium ribbon. The reaction is represented by the equation
This equation can be obtained by combining equations (1), (2), and (3):
The pre-lab portion of this experiment requires you to combine equations (1), (2), and (3) to obtain equation (4) before you do the experiment. Heats of reaction for equations (1) and (2) will be determined in this experiment. As you may already know, ΔH for reaction (3) is –285.8 kJ.
Objectives
In this experiment, you will
- Combine three chemical equations to obtain a fourth.
- Use prior knowledge about the additivity of reaction heats.
- Determine the heat of combustion of magnesium ribbon.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #19 of Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


