Endothermic and Exothermic Reactions
Experiment #5 from Physical Science with Vernier
- Subject
- Physical Science
Introduction
Some chemical reactions absorb energy and are called endothermic reactions. Many chemical reactions give off energy. Chemical reactions that release energy are called exothermic reactions. You will study one endothermic reaction and one exothermic reaction in this experiment.
In Part I, you will study the reaction between citric acid solution and baking soda. An equation for the reaction is
In Part II, you will study the reaction between magnesium metal and hydrochloric acid. An equation for this reaction is
Objectives
In this experiment, you will
- Observe two chemical reactions.
- Measure temperature changes.
- Determine the change in temperature, Δt, for each of the reactions.
- Identify endothermic and exothermic reactions.
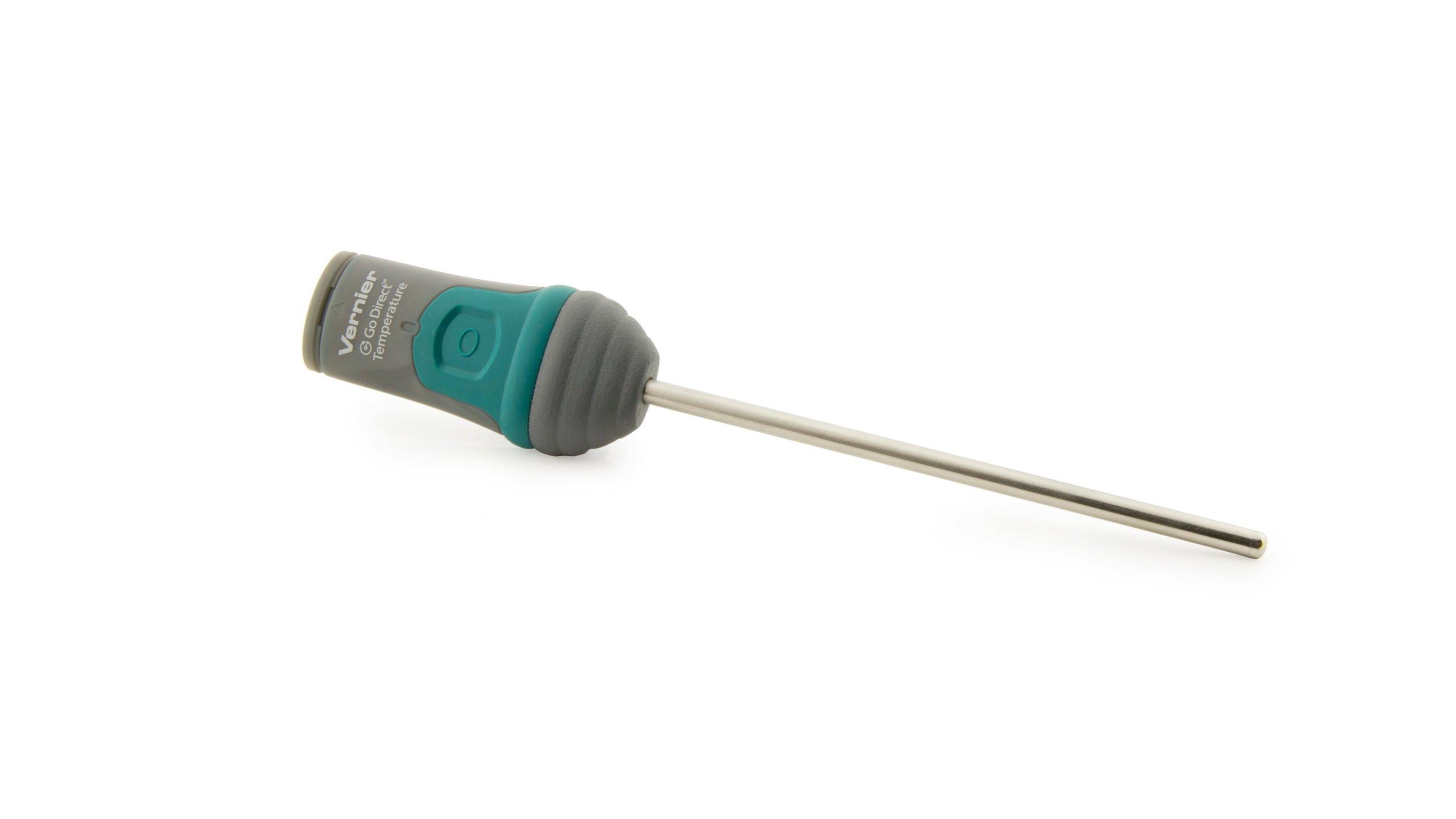
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #5 of Physical Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


