Instrumentation is used in the undergraduate chemistry curriculum to help demonstrate the fundamental aspects of chemical reactions and demonstrate how it can be used to determine certain properties of a chemical system. For example, absorbance spectroscopy teaches students about transmission and absorption of radiation by a compound and how these measurements can be used to determine concentration or chemical reaction order. Chromatography illustrates to students how the structure of compounds can help isolate them from others. When certain techniques are coupled together, the concepts are layered and even more can be learned about the system being studied.
Flash photolysis spectroscopy is a type of time-resolved absorbance spectroscopy that helps students investigate chemical reaction order as well as the basics of photochemistry. Flash photolysis is often referred to as a “pump-probe technique” because it involves an excitation source or a “pump” and a detection source or a “probe”. This technique was so groundbreaking that the 1969 Nobel Prize in Chemistry was awarded to the scientists who developed it.
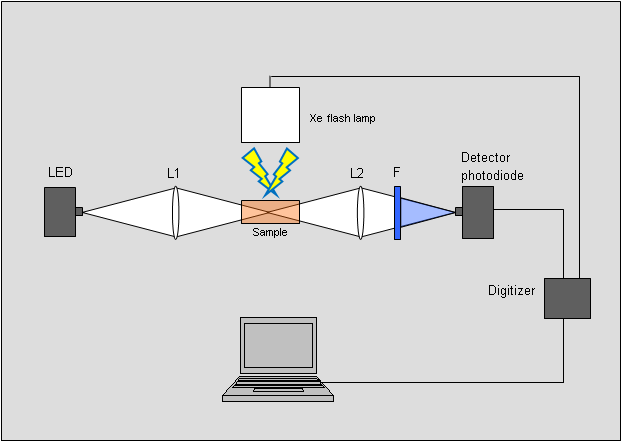
The diagram below shows a typical flash photolysis setup. In this system, white light from an LED light source probes any spectral changes made in the system by the excitation light pulse. A xenon flash lamp provides the photo-excitation pulse. The white light from the LED source is focused on the sample. From the sample this beam goes through a wavelength filter as it is focused on a photodiode, which detects this light’s intensity. When the xenon lamp is flashed, an intense near-UV, white light pulse enters the sample. If it causes changes in the absorption of the sample at the filter’s wavelength, the detector measures these changes. The voltage from the detector is collected, digitized, and stored as a function of time.
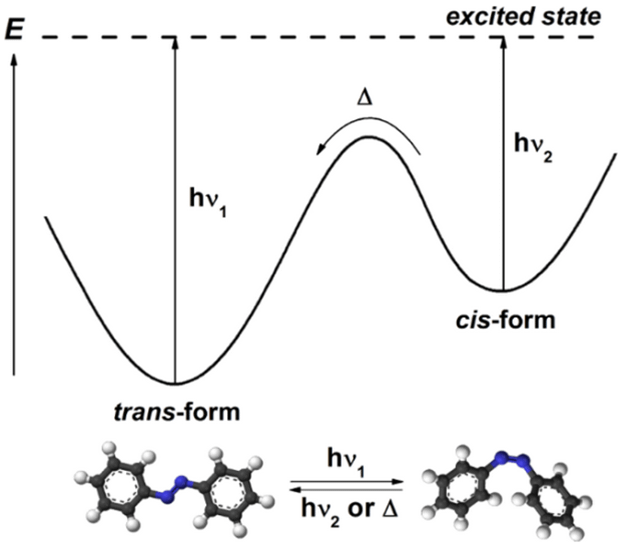
With recent advances in photochemistry in a number of disciplines, understanding photo-induced chemical kinetics is quickly becoming an essential part of the undergraduate chemistry curriculum. Due to limitations in affordable instrumentation, photochemical kinetics is often left to the textbook alone. The Vernier Flash Photolysis Spectrometer is an affordable option available to instructors to help students get hands-on experience with this important technique. We provide a number of free experiments to get you started, including one that involves exploration of a simple light-induced, cis-trans isomerization of Congo Red. Congo Red is a diazo dye that is a derivative of azobenzene. When light excites the ground state trans- form at its visible broadband absorbance, some ground state molecules are converted to a higher energy cis- form instantaneously (on this time scale, at least). The cis- state is metastable with respect to the trans- ground state resulting in slow conversion back to this trans- ground state, as shown in the state diagram below. The loss of the absorbance at 600 nm observed by the Vernier Flash Photolysis Spectrometer gives students the opportunity to follow the progress of a thermal cis-trans isomerization and measure its rate on timescales that cannot be achieved by traditional mixing methods.
The data and analysis provides an opportunity for discussion with students about various topics, including perturbation kinetics, photochemistry, fast kinetics, and bimolecular rate constants. This experiment, and others like it, allow for easy incorporation of time-resolved spectroscopy into the undergraduate physical chemistry, biochemistry, organic chemistry, and inorganic chemistry curriculums.
