Experiments
Here are experiments our science specialists have selected to support the IB* topic.
Energy in Food
Experiment #1 from Biology with Vernier
In this experiment, you will
- Measure temperature changes.
- Monitor the energy given off by food as it burns.
- Determine and compare the energy content of different foods.
Investigating Protein
Experiment #5 from Investigating Biology through Inquiry
In the Preliminary Activity, you will use a spectrophotometer and the Bradford protein assay to determine the protein content of milk. The Bradford protein assay is an extremely sensitive assay for protein. The Bradford reagent contains a dye called Coomassie G-250 that can interact with the R‑group of specific amino acids. The dominant protein in milk is called casein, which is composed of 224 amino acids. Thirteen of these amino acids react with the dye in the Bradford reagent. These amino acids include one tryptophan, four arginines, four tyrosines, and four histidines.
When the dye in the Bradford reagent interacts with these specific amino acids it turns the solution blue. The greater the concentration of protein in solution the deeper the color will be. If a set of known protein concentrations are allowed to react with a known concentration of Bradford reagent, we can measure the absorbance of the resulting solutions to create a standard curve. When a graph of absorbance vs. concentration is plotted for the standard solutions, a direct relationship should result. The direct relationship between absorbance and concentration for a solution is known as Beer’s law. To determine the protein concentration of an unknown solution, we can measure its absorbance and see where it falls on the standard curve. Because the relationship is linear, we could also calculate the protein concentration using the formula for the standard curve.
After completing the Preliminary Activity, you will first use reference sources to find out more about protein before you choose and investigate a researchable question. Some topics to consider in your reference search are:
- macromolecule
- protein
- monomer
- polymer
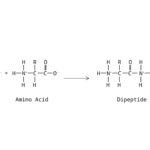
- amino acid
- alpha carbon
- carboxyl group
- primary structure
- amino group
- R group
- peptide bond
- polypeptide
- Bradford protein assay
- Beer’s law
- Educational Standard
- International Baccalaureate (IB)
- Subject
- Sports, Exercise, and Health Science
- Section
- Core
- Topic
- 3. Energy Systems
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
