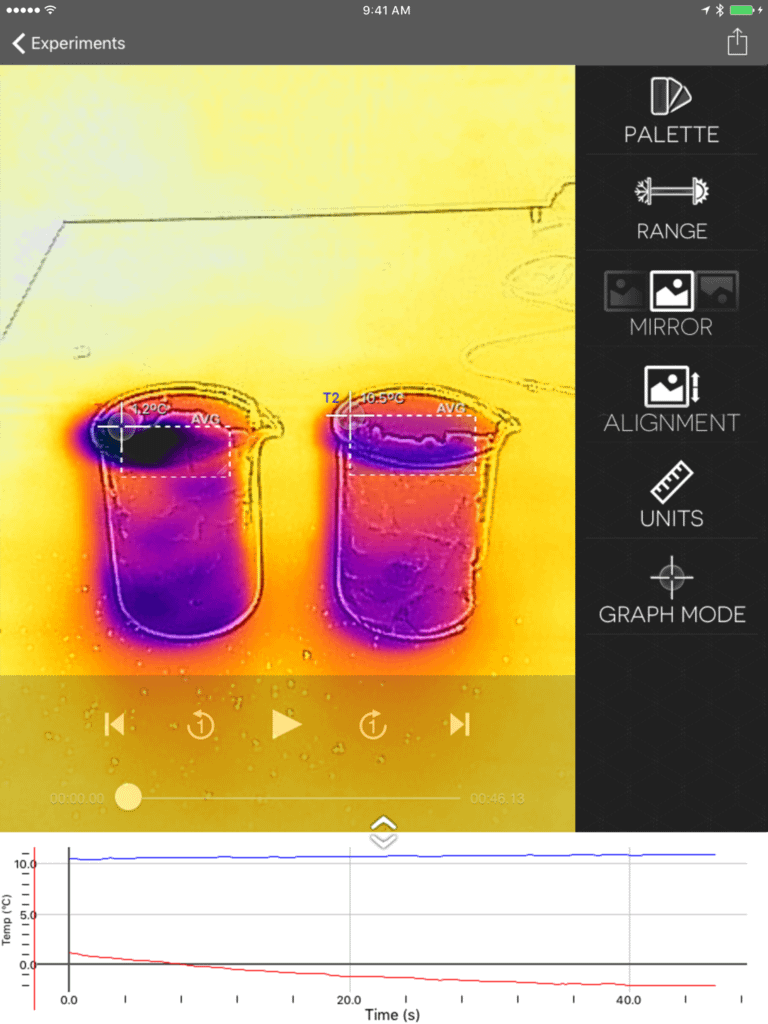
Observe freezing point depressions using beakers filled with ice and with ice and salt. Adding salt to ice lowers the temperature compared to ice alone, thus lowering the freezing point of water. The sample data above shows ice with salt (left) and ice alone (right). The ice with salt has a much lower temperature, even going below 0°C.
