Boyle’s Law: Pressure-Volume Relationship in Gases
Experiment #6 from Chemistry with Vernier
- Education Level
- High School
- Subject
- Chemistry
Video Overview
Introduction
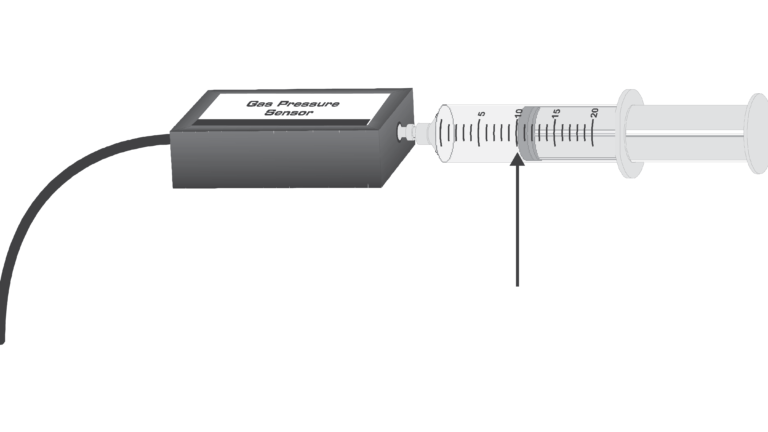
The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we use will be air, and it will be confined in a syringe connected to a Gas Pressure Sensor. When the volume of the syringe is changed by moving the piston, a change occurs in the pressure exerted by the confined gas. This pressure change will be monitored using a Gas Pressure Sensor. It is assumed that temperature will be constant throughout the experiment. Pressure and volume data pairs will be collected during this experiment and then analyzed. From the data and graph, you should be able to determine what kind of mathematical relationship exists between the pressure and volume of the confined gas. Historically, this relationship was first established by Robert Boyle in 1662 and has since been known as Boyle’s law.
Objectives
In this experiment, you will
- Use a Gas Pressure Sensor and a gas syringe to measure the pressure of an air sample at several different volumes.
- Determine the relationship between pressure and volume of the gas.
- Describe the relationship between gas pressure and volume in a mathematical equation.
- Use the results to predict the pressure at other volumes.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Correlations
Teaching to an educational standard? This experiment supports the standards below.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #6 of Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

