Introduction
The evaporation of a liquid absorbs energy and cools its surroundings. Such a process is said to be endothermic. You have probably experienced this phenomenon as you stepped from a swimming pool on a windy day. A major factor in determining the rate of evaporation and the resulting cooling is the strength of attraction between the molecules of a liquid. Substances with strong attractions between molecules evaporate slowly and cool slightly during evaporation. In this experiment, you will study temperature changes caused by the evaporation of different alcohols.
Objectives
- Draw structural formulas for alcohols.
- Measure temperatures as three alcohols evaporate.
- Determine the change in temperature, Δt, for each of three alcohols.
- Predict the change in temperature, Δt, for a fourth alcohol.
- Determine the change in temperature, Δt, for the fourth alcohol.
- Graph the results.
- Use the results to make conclusions about intermolecular attractions.
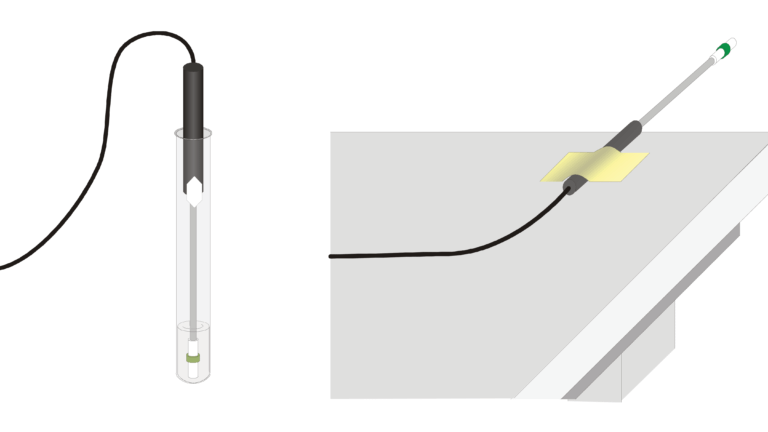
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #4 of Physical Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


