Experiments
Here are experiments our science specialists have selected to support this IB* topic.
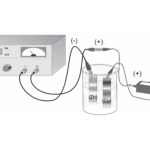
Determining Avogadro's Number
Experiment #31 from Advanced Chemistry with Vernier
In this experiment, you will
- Prepare an electrochemical cell to oxidize a copper electrode.
- Measure the amount of copper that was deposited in the electroplating process and determine the average current used.
- Calculate a value for Avogadro’s number and compare it to the accepted value.
- Educational Standard
- International Baccalaureate (IB) 2025
- Subject
- Chemistry
- Organizing concepts
- Structure 1. Models of the particulate nature of matter
- Subtopics
- Structure 1.4—Counting particles by mass: The mole
- Level
- Standard level and higher level
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
