Oxidation-Reduction Titrations
Experiment #19 from Investigating Chemistry through Inquiry
- Subject
- Chemistry
Introduction
A titration, as you recall, is a convenient method of learning more about a solution by reacting it with a second solution of known molar concentration. There are a number of ways to measure the progress of a titration. In this experiment, you will use an ORP (Oxidation-Reduction Potential) Sensor to measure the electrical potential of the reaction being studied in a titration.
You will be titrating commercial bleach with hydrogen peroxide of known molarity in order to determine the molarity of the bleach sample. The reaction being studied can be represented as follows:
where NaOCl is sodium hypochlorite, a representative component of bleach, and H2O2 is the formula for hydrogen peroxide. The products are sodium chloride, water, and oxygen. Your sample of H2O2 will come from a bottle of ordinary, over-the-counter hydrogen peroxide purchased at a grocery or a drug store. The concentration of this product is labeled as 3% mass/volume, which is ~0.88 M.
Objectives
In the Preliminary Activity, you will be titrating commercial bleach with hydrogen peroxide of known molarity in order to determine the molarity of the bleach sample.
After completing the Preliminary Activity, you will first use reference sources to find out more about oxidation, reduction, and oxidation-reduction titrations before you choose and investigate a researchable question using oxidation-reduction titrations.
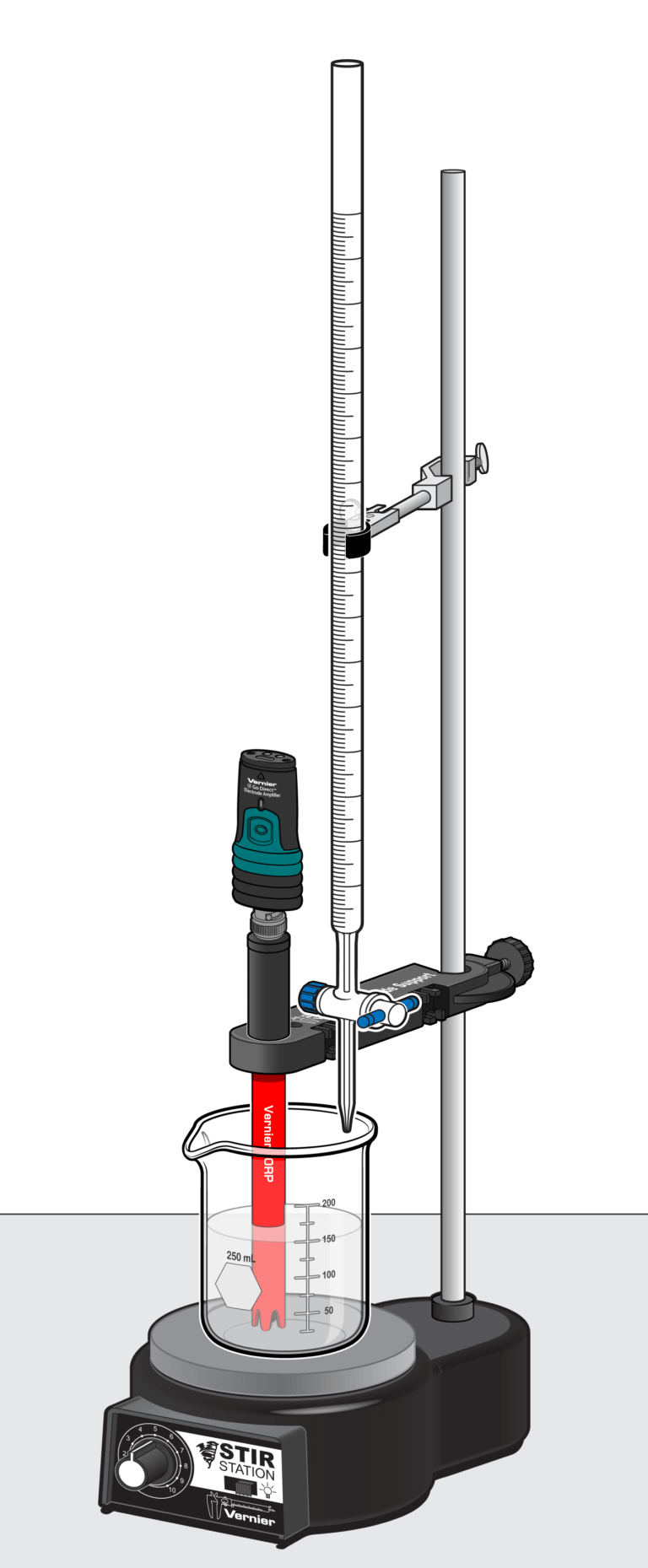
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #19 of Investigating Chemistry through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

