Introduction
More than 70% of the Earth’s surface is covered by water. Yet people in some cities and even countries do not always have enough fresh water to drink. How can this be? The answer is that most of the Earth’s water is undrinkable because it contains high concentrations of salts and other minerals. Water’s saltiness is referred to as its salinity. Ocean water has such a high salinity that we would actually become sick and dehydrated by drinking it. To make ocean water drinkable, the salts and minerals must be removed through a process called desalination.
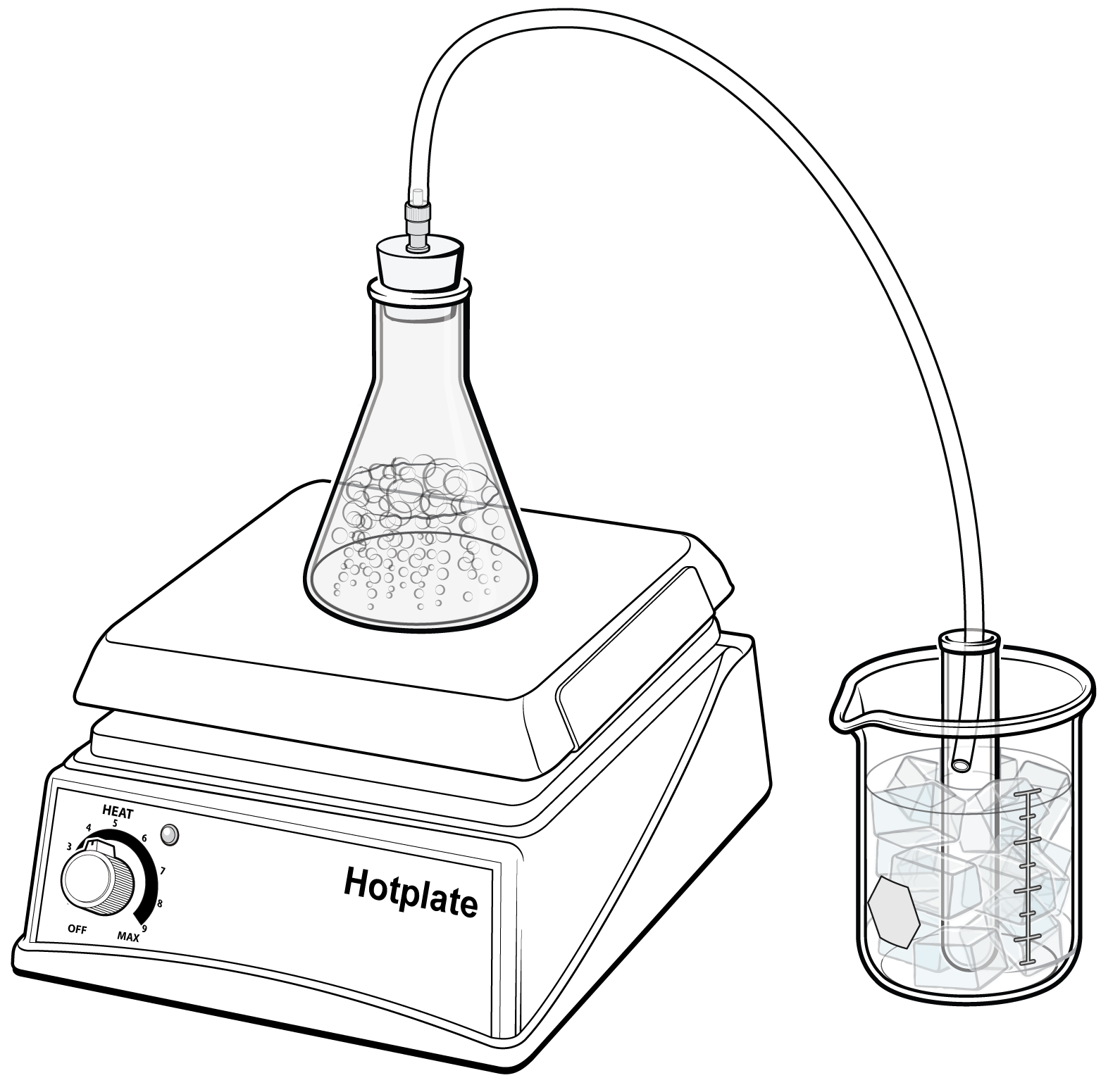
One method of desalination is called distillation. Distillation involves heating the salty water so that it vaporizes. As the water vaporizes, it leaves the salt and other minerals behind. The water vapor is then cooled and condensed back into liquid water that is now much less salty than it was before. This process is expensive because it requires a lot of energy to heat the water, but for some communities, desalination is the only option.
In this experiment, you will first use a conductivity probe to measure the salinity of a sample of saltwater. You will distill the sample water by heating it and collecting the condensed vapor. After that, you will use the conductivity probe to measure the salinity of the condensed vapor in order to determine the amount of salt removed during distillation.
Objectives
- Use a conductivity probe to measure the salinity of water before and after desalination.
- Distill saltwater.
- Calculate the amount of salt removed from the sample.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #18 of Earth Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.