Introduction
Energy content is an important property of food. The energy your body needs for running, talking, and thinking comes from the food you eat. Energy content is the amount of heat produced by the burning of 1 gram of a substance, and is measured in joules per gram (J/g).
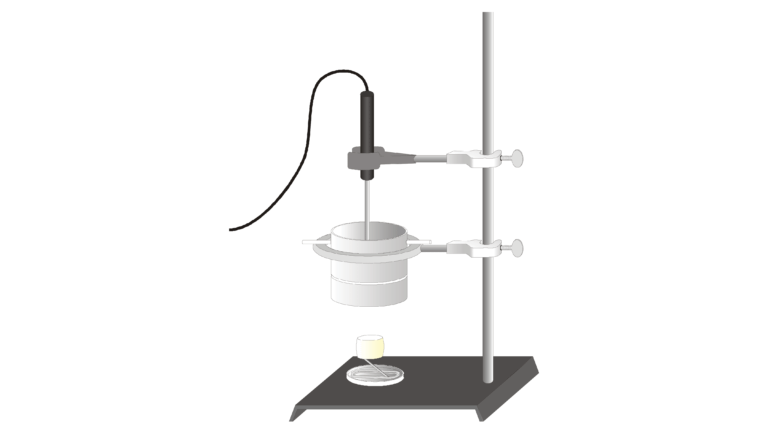
You can determine energy content by burning a portion of food and capturing the heat released to a known mass of water in a calorimeter. If you measure the initial and final temperatures, the energy released can be calculated using the equation
where H = heat energy absorbed (in J), Δt = change in temperature (in °C), m = mass (in g), and Cp = specific heat capacity (4.18 J/g°C for water). Dividing the resulting energy value by grams of food burned gives the energy content (in J/g).
Objectives
In this experiment, you will
- Measure temperature.
- Analyze data.
- Use a balance.
- Determine energy content.
- Compare the energy content of different foods.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #10 of Physical Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


