Go Direct® Ammonium Ion-Selective Electrode BNC User Manual
Order Code: GDX-NH4-BNC
The Go Direct Ammonium Electrode BNC is used to measure the concentration of ammonium (NH4+) ions in aqueous samples. It is designed to be used with the Go Direct Ion-Selective Electrode Amplifier (order code GDX-ISEA).
Note: Vernier products are designed for educational use. Our products are not designed nor are they recommended for any industrial, medical, or commercial process such as life support, patient diagnosis, control of a manufacturing process, or industrial testing of any kind.
What's Included
- Go Direct Ammonium Electrode BNC, packed in a storage bottle with a damp sponge
- 30 mL bottle of High Standard solution with SDS (100 mg/L NH4+)
- 30 mL bottle of Low Standard solution with SDS (1 mg/L NH4+)
- Short-Term ISE Soaking Bottle
Using the Product
To prepare the electrode to make measurements, follow this procedure:
- Connect the Ion-Selective Electrode BNC to the Go Direct Ion-Selective Electrode Amplifier. Push the BNC connector of the electrode onto the connector on the amplifier, then turn the BNC connector about one-half turn clockwise.
- Connect the amplifier to your computer, Chromebook™, LabQuest®, or mobile device and run the data-collection software. Change the sensor channel to the appropriate ion or Potential, if necessary.
- Your ISE needs to be prepared before use. This includes a 30-minute soak in the High Standard solution.
- If you plan to use the electrode outside the range of the standards provided, you will need to prepare your own standards and use those for soaking and calibration.
- The ISE should not rest on the bottom of the container.
- The small white reference contacts near the tip of the electrode should be immersed.
- Make sure no air bubbles are trapped below the ISE.
- Do not leave the ISE soaking for more than 24 hours.
- Do not completely submerge the sensor. The BNC connection is not waterproof.
Preparing the Ammonium ISE for Use
Soak the electrode in the High Standard solution (included with the ISE) for approximately 30 minutes. The ISE should not rest on the bottom of the container, and the small white reference contacts near the tip of the electrode should be immersed. Make sure no air bubbles are trapped below the ISE. Important: Do not leave the ISE soaking for more than 24 hours. Important: If you plan to use the electrode outside the range of the standards provided, you will need to prepare your own standards and use those for soaking.
Note: If the ISE needs to be transported to the field during the soaking process, use the Short-Term ISE Soaking Bottle. Remove the cap from the bottle and fill it 3/4 full with High Standard. Slide the bottle’s cap onto the ISE, insert it into the bottle, and tighten.
For long term storage, greater than 24 hours, make sure the sensor is stored in its storage bottle with the sponge slightly damp.
Collecting Data
- Remove the storage bottle from the soaking solution (high standard). Thoroughly rinse the lower section of the probe, especially around the tip, using distilled or deionized water. Blot dry with a paper towel.
- Insert the tip of the ISE into the aqueous sample to be tested. Important: Make sure the ISE is not resting on the bottom of the container, the white reference contacts near the tip of the electrode are immersed, and no air bubbles are trapped below the ISE. Note: Do not completely submerge the sensor.
- Hold the ISE still until the reading stabilizes and record the displayed reading. Note: With some aqueous samples, especially those at high concentrations, it could take several minutes for the reading of the Ammonium ISE to stabilize. If you know the approximate concentrations of your samples, it is best to analyze them from lowest concentration to highest.
Note: Readings are reported in mV by default. If you choose to change the sensor channel to ammonium concentration, you may want to calibrate the sensor. If you wish to convert the mV reading to concentration manually, refer to the How the Sensor Works section of this user manual.
Calibration
A calibration is stored on each sensor before it is shipped. As the membrane ages, this factory calibration may become inadequate. For best results, we recommend performing a two-point calibration.
Note: If you plan to use the electrode outside the range of the standards provided, you will need to prepare your own standards and use those for soaking and calibration. Standards should be two decades apart (e.g., 5 mg/L and 500 mg/L).
For additional calibration information, see www.vernier.com/til/4011
Specifications
|
Range (mV) |
–1000 mV to +1000 mV (GDX-EA) |
|
Range (concentration) |
1 to 18,000 mg/L (or ppm) |
|
Reproducibility (precision) |
±30 mV |
|
Interfering ions |
K+, Li+, Na+, Cs+, Mg2+, Ca2+, Sr2+, Ba2+ |
|
pH range |
2–7 (no pH compensation) |
|
Temperature range |
0–40°C (no temperature compensation) |
|
Electrode slope |
+56 ±3 mV/decade at 25°C |
|
Electrode resistance |
0.1 to 5 MΩ |
|
Minimum sample size |
must be submerged 2.8 cm (1.1 in) |
Care and Maintenance
Short-term wet storage (less than 24 hours): Fill the Short-Term ISE Soaking bottle 3/4 full with High Standard. Loosen the cap, insert the electrode into the bottle, and tighten.
Long-term storage of the ISE (longer than 24 hours): Moisten the sponge in the bottom of the long-term storage bottle with distilled water. When you finish using the ISE, rinse it off with distilled water and blot it dry with a paper towel. Loosen the lid of the long-term storage bottle and insert the ISE. Note: The tip of the ISE should NOT touch the sponge. Also, make sure the white reference mark is inside the bottle. Tighten the lid. This will keep the electrode in a humid environment, which prevents the reference junctions from completely drying out.
Maintaining and Replacing the ISE Standard Calibration Solutions
Having accurate standard solutions is essential for performing good calibrations. The two standard solutions that were included with your ISE can last a long time if you take care not to contaminate them. At some point, you will need to replenish your supply of standard solutions. Vernier sells replacement standards in 500 mL volumes. Order codes are
- NH4-LST: Ammonium Low Standard, 1 mg/L
- NH4-HST: Ammonium High Standard, 100 mg/L
To prepare your own standard solutions, use the information in the table below. Note: Use glassware designed for accurate volume measurements, such as volumetric flasks or graduated cylinders. All glassware must be very clean.
|
Standard Solution |
Concentration (mg/L or ppm) |
Preparation Method Using High Quality Distilled Water |
|---|---|---|
|
Ammonium High Standard |
100 mg/L NH4+ as N |
0.382 g NH4Cl / 1 L solution |
|
Ammonium Low Standard |
1 mg/L NH4+ as N |
Dilute the High Standard by a factor of 100 (from 1000 mg/L to 10 mg/L).* |
*Perform two serial dilutions as described below.
- Combine 100 mL of the High Standard with 900 mL of distilled water. Mix well.
- Combine 100 mL of the solution made in the previous step with 900 mL of distilled water. Mix well.
Ammonium ISE Replacement Membrane Modules
The Go Direct Ammonium Ion-Selective Electrode BNC has a PVC membrane with a limited life expectancy. It is warranted to be free from defects for a period of 12 months from the date of purchase; it is possible, however, that you may get somewhat longer use than the warranty period. If you start to notice a reduced response, it is probably time to replace the membrane module. Important: Do not order membrane modules far in advance of the time you will be using them; the process of degradation takes place even when they are stored on the shelf.
How the Sensor Works
Combination Ion-Selective Electrodes consist of an ion-specific (sensing) half-cell and a reference half-cell. The ion-specific half-cell produces a potential that is measured against the reference half-cell depending on the activity of the target ion in the measured sample. The ion activity and the potential reading change as the target ion concentration of the sample changes. The relationship between the potential measured with the ISE and the ion activity, and thereby the ion concentration in the sample, is described by the Nernst equation:
- E = measured potential (mV) between the ion-selective and the reference electrode
- Eo = standard potential (mV) between the ion-selective and reference electrodes
- R = universal gas constant (R = 8.314 J mol-1 K-1)
- T = temperature in K (Kelvin), with T (K) = 273.15 + t °C where t is the temperature of the measured solution in °C.
- F = Faraday constant (96485 C mol-1)
- n = valence of the ion
- C = concentration of ion to be measured
- Co = detection limit
Since R and F are constant, they will not change. The electrical charge of the ion (valence) to be measured is also known. Therefore, this equation can be simplified as:
E = Eo –S • log(C + Co)
where is the ideal slope of the ISE.
The following table describes ideal behavior:
| Ion Examples | n (valence of ion) | S (at 25 °C), mV/decade |
|---|---|---|
| Calcium (Ca2+) | +2 | +29.58 |
| Potassium (K+), Ammonium (NH4+) | +1 | +59.16 |
| Nitrate (NO3-), Chloride (Cl-) | –1 | –59.16 |
Assuming C0 is near zero, the equation can be rewritten as:
C = 10˄[(E – Eo) / S]
allowing for the calculation of the ion concentration.
It is very important to note that this table reflects ideal behavior. Ion-selective electrodes have slopes that are typically lower than ideal. It is generally accepted that an ISE slope from 88–101% of ideal is allowable. The slope (S) is an indicator of ISE performance. If the slope changes significantly over time, it may indicate that it is necessary to replace the ISE sensor tip.
Convert Potential to Concentration (Optional)
To measure the mV readings from an aqueous sample, calibration is not required. To convert mV readings to concentration (mg/L or ppm), you will use a modified version of the Nernst Equation:
C = 10˄[(E – Eo) / Sm]
C = concentration of ion to be measured (mg/L or ppm)
E = measured potential of sample (mV)
Eo = measured potential (mV) at a C = mg/L NH4+ –N concentration
Sm = measured electrode slope in mV/decade
The value of Sm, the measured electrode slope, is determined by measuring the potential of two standard solutions, and solving the equation below:
Sm = – [(Low Standard – High Standard) / # of decades*]
*A decade is defined as the factor of the difference between the two standard solutions. For example, the difference between a 1mg/L standard and a 100 mg/L standard is 2 decades (a factor of 100 difference, or 1 × 102).
Example Calculation, converting mV to mg/L
For this example, the measured quantities are shown in the chart below:
| Solution | Measured Potential |
|---|---|
| 1 mg/L NH4+ standard | 0 mV |
| 100 mg/L NH4+ standard | 116 mV |
| unknown sample | 8 mV |
C = 10^[(88 mV – 0 mV)/ 58 mV/decade] = 33 mg/L NH4+–N
Units of Ammonium Concentration
Concentrations of ammonium are often expressed in units of mg/L NH4+ as N, or NH4+-N, also known as “ammonium-nitrogen”. This means that the concentration of ammonium is expressed with regard to the element nitrogen alone. The standards that are included with your Ammonium ISE have concentrations of 1 and 100 mg/L NH4+-N. Here is the calculation for a 100 mg/L NH4+-N standard solution that is prepared by adding solid NH4Cl to distilled water:
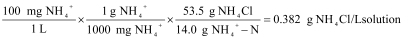
To convert concentration from NH4+-N to NH4+ you will refer to the percent composition of nitrogen in an ammonium ion, as shown below using
100 mg/L NH4+-N as an example:

Troubleshooting
Ammonium in the Environment
The Ammonium Ion-Selective Electrode (ISE) can be used to determine concentrations of NH4+ ions in aqueous solutions, in units of mg/L, ppm, or mol/L. Concentrations of aqueous ammonium ions should not be mistaken for concentration of aqueous ammonia, or NH3(aq). The concentrations of these two species, though different, are often involved in the same equilibrium reaction:
Reaction 1: NH3(aq) + H+(aq) ↔ NH4+ (aq)
In a more acidic environment, higher concentrations of H+ ions will cause this reaction to shift toward the right, resulting in higher concentrations of NH4+. In a more basic (alkaline) environment, the concentration of NH4+ will be lower, causing the reaction to shift toward the reactants, producing higher concentrations of NH3. At pH values greater than 10, most of the ammonium ions will be converted to ammonia. At pH values less than 7.5, most of the aqueous ammonia will be converted to ammonium ions.
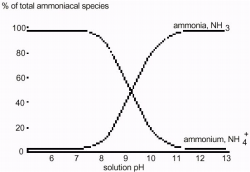
Freshwater Samples for Ammonium Concentration
While permissible levels of ammonium in drinking water should not exceed 0.5 mg/L, streams or ponds near heavily fertilized fields may have higher concentrations of this ion. Fertilizers containing ammonium sulfate, (NH4)2SO4, or ammonium nitrate, NH4NH3, may result in runoff from fields containing higher levels of the ammonium ion, NH4+. Monitoring ammonium levels on a stream that borders fertilized fields may show significant seasonal differences in NH4+ concentrations. In this kind of study, you may also take pH measurements in your water samples; as indicated in the previous paragraph, higher or lower pH values can greatly affect the ratio of NH4+ / NH3 in a sample. Since the Ammonium ISE measures only NH4+ levels, you may want to adjust your samples to the same pH value each time you make measurements; this may not be necessary if you have relatively “hard” water. Hard water is naturally buffered against changes in pH.
Using Ionic Strength Adjustor (ISA) Solutions to Improve Accuracy
For optimal results at low concentrations of ions, a standard method for making measurements with ion-selective electrodes is to add ionic strength adjustor (ISA) solutions to each of your standard solutions and samples.
Adding an ISA ensures that the total ion activity in each solution being measured is nearly equal, regardless of the specific ion concentration. This is especially important when measuring very low concentrations of specific ions. The ISA contains no ions common to the ISE itself. Note: The addition of ISA solutions to samples or standards does not need to be highly accurate. You can add the ISA solution dropwise to a sample using a disposable plastic Beral pipet. We recommend using 0.25 M magnesium acetate solution prepared in 0.5 M acetic acid solution as the ISA for the Ammonium ISE. To prepare this solution, dissolve 53.6 grams of magnesium acetate in sufficient 0.5 M acetic acid solution to make 1.0 liter. Commonly, ISA is added in a 1:50 ratio, or 1 mL of ISA added to 50 mL of water to be tested.
See General tips for using Ion Selective Electrodes at www.vernier.com/til/665
Repair Information
If you have followed the troubleshooting steps and are still having trouble with your Go Direct Ammonium Electrode BNC, contact Vernier Technical Support at support@vernier.com or call 888-837-6437. Support specialists will work with you to determine if the unit needs to be sent in for repair. At that time, a Return Merchandise Authorization (RMA) number will be issued and instructions will be communicated on how to return the unit for repair.
Accessories/Replacements
| Item | Order Code |
|---|---|
|
BTL |
|
|
NH4-HST |
|
|
NH4-LST |
|
|
NH4-MOD |
|
|
GDX-ISEA |
Warranty
Warranty information for this product can be found on the Support tab at www.vernier.com/gdx-nh4-bnc/#support
General warranty information can be found at www.vernier.com/warranty
Additionally, the warranty does not cover accidental breakage of the glass bulb of the pH sensor.
Contact Support
Fill out our online support form or call us toll-free at 1-888-837-6437.

