Many schools can’t afford a classroom set of spectrophotometers. So how can you use a single spectrophotometer and a class set of colorimeters to have your students discover the best wavelength of light to use in an experiment?
For Beer’s law and kinetics experiments, students must choose a specific wavelength to conduct the experiment. Students are expected to understand and explain why they would choose one wavelength over another.
We’ve written an experiment that allows you to do just that. The Vernier Colorimeter has four LEDs that provide wavelengths of 430 nm, violet light; 470 nm, blue light; 565 nm, yellow light; and 635 nm, red light. Using a colored solution, your students will test each wavelength on their Colorimeter. They will calibrate the Colorimeter and record the absorbance value of the solution at each wavelength. Afterward, they will compare the absorbance at each wavelength.
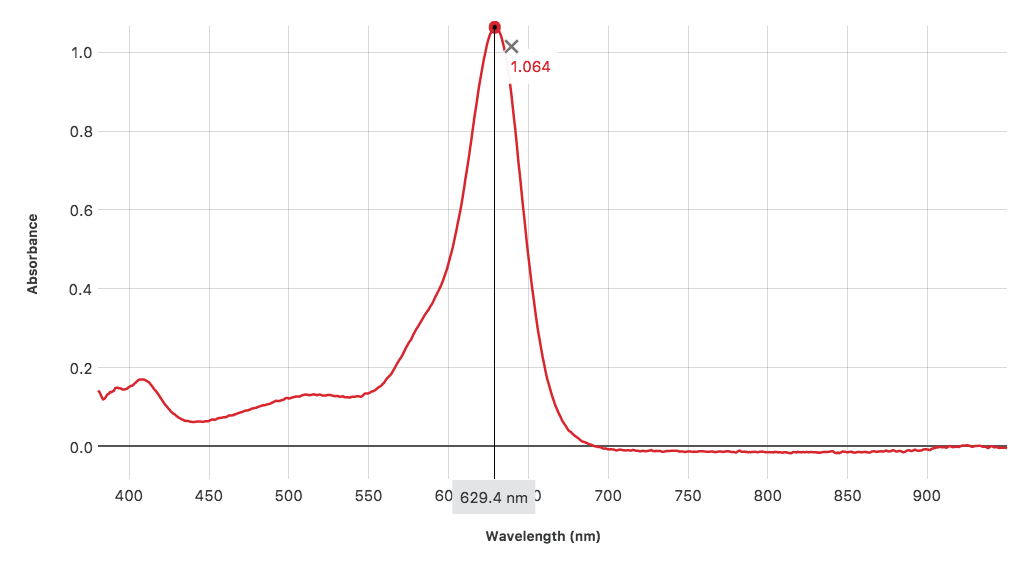
While your students are doing this, you will capture an absorbance spectrum with a spectrophotometer for the same solution that the students are using. They will then compare their results with the spectrum you project to the class. The discussion that follows should help your students understand why they would choose one wavelength, λmax, over another for a particular solution and also for a specific experiment.
