It can be challenging to teach electrochemistry to students in a way that is simple, yet engaging. No matter the challenge, its relevancy to students is evident by its reference in the 2019 AP Chemistry Exam. Students were asked to examine reduction potential half-reactions for some halogens in order to write a balanced equation and calculate Eº.
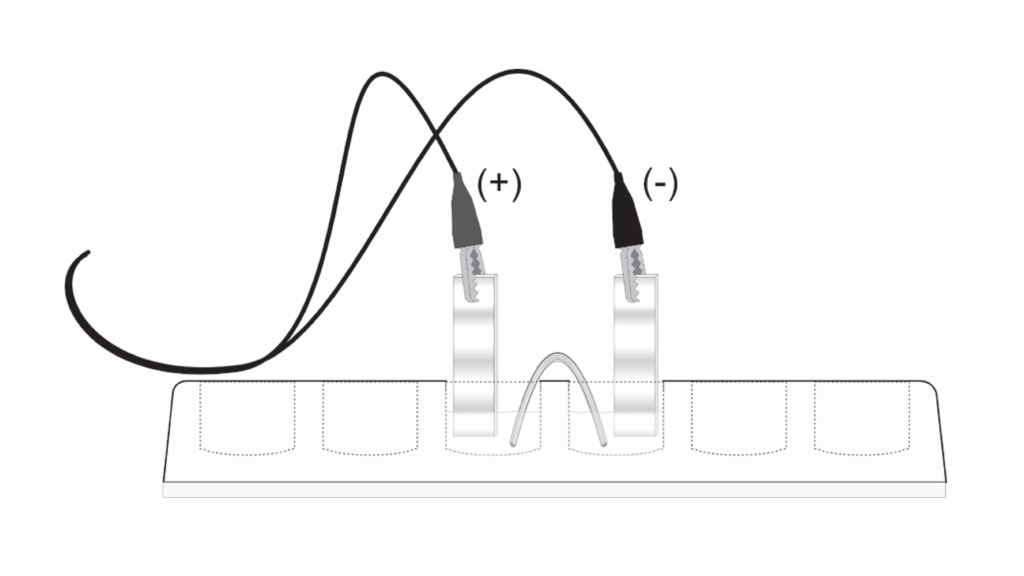
Using the Go Direct® Voltage Probe, along with our experiments, makes teaching electrochemistry less complicated and more fun. Oxidation and reduction is a basic concept when teaching electrochemistry. This is easy to explore by setting up “micro-voltaic” cells, also known as galvanic cells. Basically, students will be making batteries. Using a Go Direct Voltage Probe, a piece of filter paper, sodium nitrate as the salt bridge, and any metals and salts of these metals at your disposal, your students can build their own electromotive series. In this simple, fun activity, they will learn how to write half reactions and calculate cell potentials. Check out Experiment 28, “Establishing a Table of Reduction Potentials: Micro-Voltaic Cells” in our Chemistry with Vernier lab book for more details.
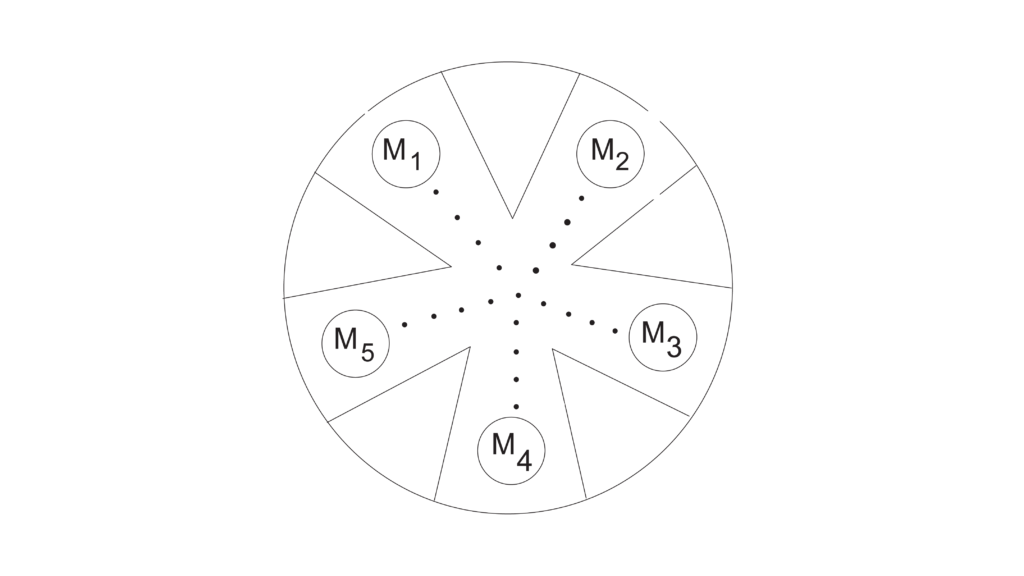
A visually more traditional half-cell set up, still considered micro-scale, would be Experiment 20, “Electrochemistry: Voltaic Cells” in Advanced Chemistry with Vernier. This experiment describes using a well plate chamber for each cell and a string soaked in potassium nitrate as the salt bridge.
Besides measuring cell potentials and establishing a series of reduction potentials, an added component of this experiment is to set up and test concentration cells and learn an application of the Nernst equation.
Don’t leave electrochemistry out of your curriculum. These simple experiments will give your students a basic introduction to oxidation and reduction as well as cell potentials.


