The temperature and the pH at which enzymes function are extremely important. Most organisms have a preferred temperature range in which they survive, and their enzymes function best within that temperature range. If the environment of the enzyme is too hot, acidic, or basic, the enzyme may irreversibly denature, or unravel, until it no longer has the shape to function properly. The enzyme catalase is found in nearly all living organisms; students can use our O2 gas sensors to quickly investigate the effect of temperature, pH, or other factors on catalase.
In traditional laboratory investigations of catalase and temperature, students measure the reaction rate of the enzyme by placing the substrate and enzyme in a reaction vessel at different temperatures. This is a potential confounding factor, as higher temperatures may denature the enzyme, leading to a decrease in reaction rate. Students don’t know whether the decrease in reaction rate was due to the temperature moving outside the enzyme’s preferred range or to denaturation of the enzyme.
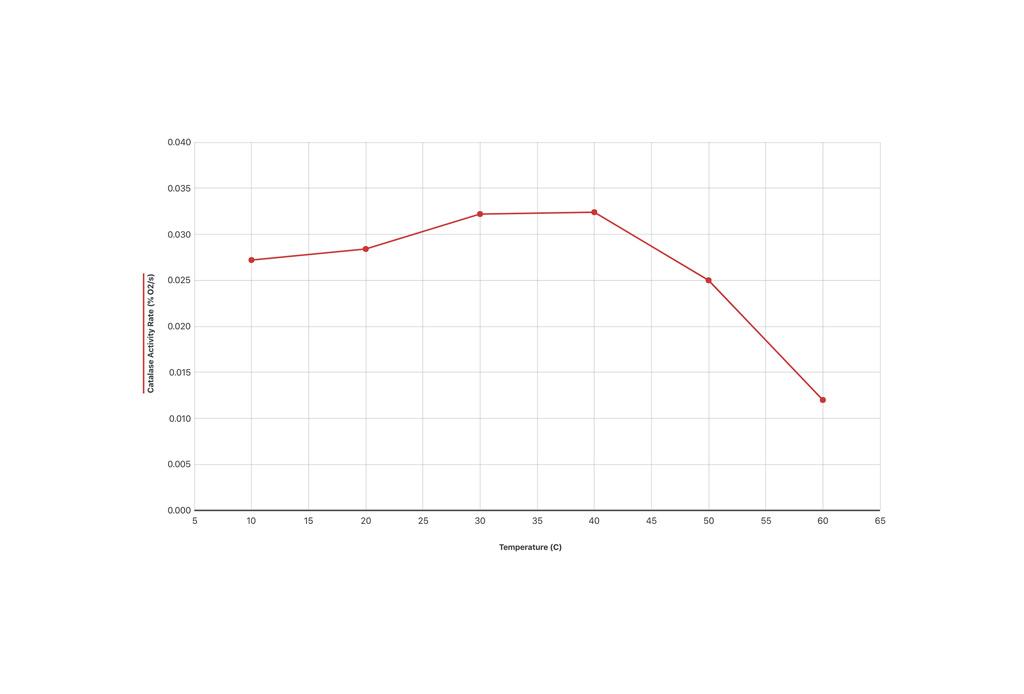
Luckily, there is a simple solution to this problem. Students can incubate a sample of the enzyme at a given temperature for 10 minutes, bring the enzyme back to room temperature, and then add the enzyme to the reaction vessel with the substrate before using an O2 gas sensor to measure the reaction rate. Following this procedure, the reaction is always performed at room temperature. As shown in the example graph, treating the enzyme to temperatures above 40°C causes a marked decrease in the reaction rate. Since the reaction occurred at room temperature, we know that the enzyme begins to denature at temperatures greater than 40°C.
Download the modified instructions on measuring the heat of denaturation using our Go Direct® O2 Gas Sensor and Graphical Analysis™ 4.

