Doug Balmer, a chemistry teacher from Warwick High School in Lititz, Pennsylvania, designed a lab experiment for his students that investigates the absorption and emission of light using a spectrophotometer. The experiment, entitled “Light Lab: Studying Light and Electrons,” is divided into four parts: understanding how a spectrophotometer works, seeing the connection between wavelength and color, observing atomic line spectra, and looking at the absorption spectra of solutions.
In the past, Balmer’s students used traditional spectroscopes to observe atomic line spectra. However, after acquiring a class set of SpectroVis® Plus Spectrophotometers and Optical Fiber accessories, his students transitioned to using these instead.
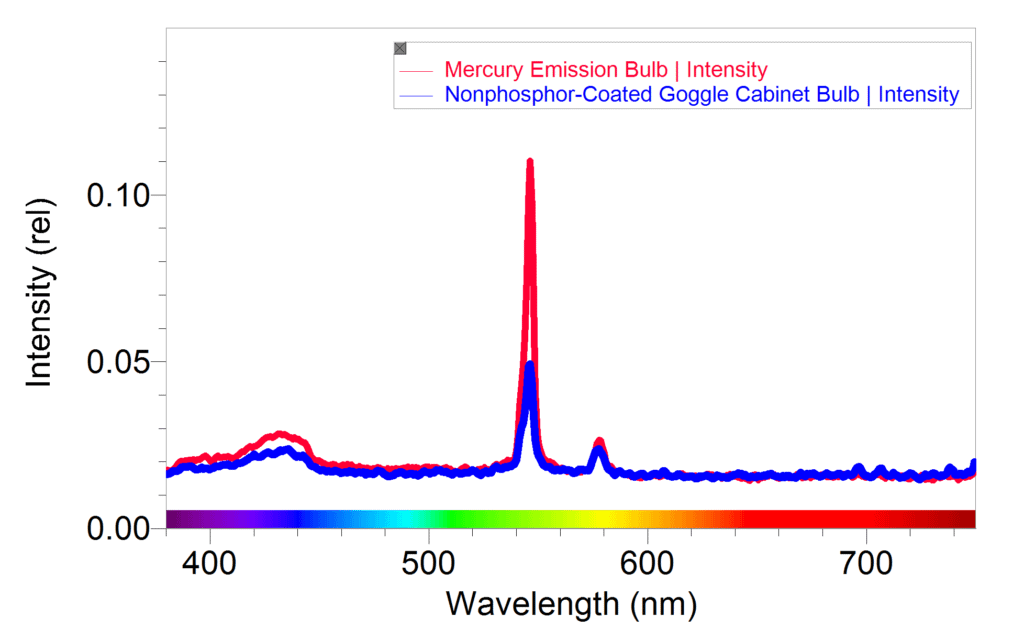
One of his objectives when observing the atomic line spectra is to determine the elements found in fluorescent light bulbs. For several years, Balmer’s students had trouble identifying mercury vapor in the bulbs. They could usually narrow it down to mercury and one other element, but the phosphorescent coating on the overhead fluorescent bulbs interfered with the results. As an alternative, he started having the students observe the spectrum of the bulbs inside their goggle cabinet. These bulbs, used to sterilize goggles, are similar to overhead fluorescent bulbs, but lack the fluorescent coating. The small dimension of the Optical Fiber allows it to be fed through the screw holes in the back of the cabinet or the cracks at the door hinges, giving students much better results when comparing the fluorescent light spectrum to the mercury spectrum tube.
