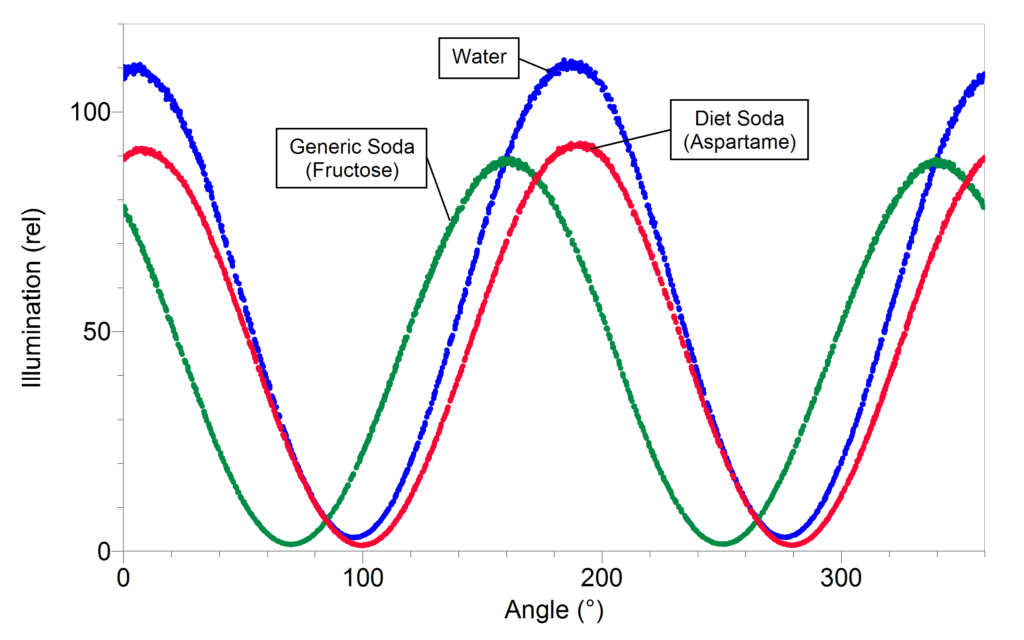
In this activity, students use the Polarimeter to identify which sugars everyday beverages have in common. First, it is necessary to concentrate the beverages. They are initially too dilute to see any differences in sugar content. One way to concentrate the solution is to dry 50 mL of each beverage over 10 g of Na2SO4 (or similar drying agent).
If you want a quantitative comparison between beverages, you should use the same volume of solution and weight of drying agent. You should also dry each solution for the same amount of time.
Once the solutions are concentrated, it is possible to see the difference between diet soda that contains aspartame (α = +15°) and regular soda that contains fructose (α = –92°). You will also be able to tell if there is a difference between “natural” sodas that may contain glucose (α = +53°) or sucrose (α = +66°) as the primary sugar and a generic soda that will contain fructose.
For college students, this can be extended to beer and wine to explore the percentage of sugar in each beverage. Clear liquids work best for these experiments, however, colored liquids can also be used.
By Melissa Hill, Ph.D.
