Chemistry has been a passion of mine since high school. Now, as an assistant professor of chemistry at Muskingum University where I also attended for my undergrad, I try to further ignite my students’ passion for the subject just as my own teachers did for me.
As a small liberal arts university, we are afforded the opportunity as educators to provide our students with a lot of one-on-one support. This is true during and after labs in both introductory and upper-level chemistry courses, as well as during the full-year senior seminars in which students work on a research-intensive project of their choosing under the close guidance of a faculty research advisor.
In the first semester of senior seminar, students read scientific literature and propose new research projects, which are reviewed by the entire chemistry faculty. In the second semester, students then carry out the proposed research where they typically spend 16 to 20 hours a week in the lab. At the end of the second semester, each student writes and presents a senior thesis.
The use of these technologies helps to build students’ proficiency using instrumentation while providing them with hands-on experience that will better prepare them for careers in the chemistry field.
The frequent use of instrumentation is central to the lab experience in senior seminar, as well as with all of my classes. This hands-on learning helps students develop skills that are critical in the real world, as applications of chemical knowledge require an understanding of instrumentation, data collection, and analysis.
This semester, one of my senior seminar students is studying how introducing polymers to commonly-used lithium sulfur batteries can slow down degradation and prevent combustion of the sulfur electrode. The student is using a spectrophotometer and Logger Pro to collect data on the absorbance spectra of lithium polysulfide and analyzing how the absorbance decreases as different polymers are added to the solution. Based on this research, the student will be able to potentially offer up a solution to this polysulfide dissolution problem, which affects many of these types of batteries and reduces battery performance.
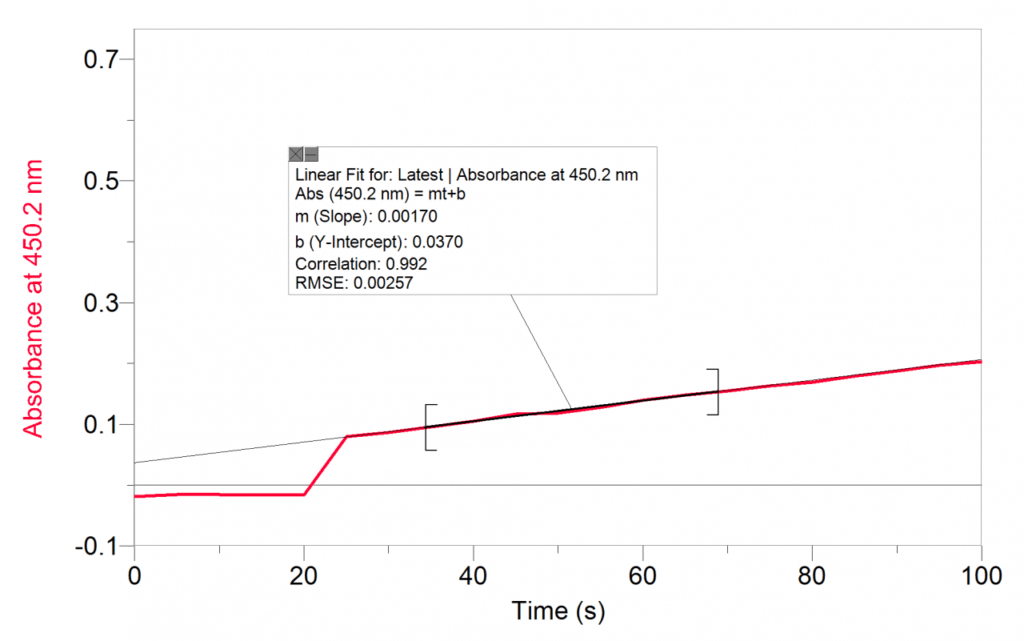
As highlighted in the 2019 Vernier/NSTA Awards submission, Vernier data-collection technology, specifically the Vernier Spectrometer or Go Direct® SpectroVis Plus, is also used with my upper-level physical chemistry students to teach them about the oxidation of iodide by iron (III) ions. In addition to determining the rate expression, students use the initial rates method to determine which step in the proposed mechanism is rate determining, based upon proposed elementary steps of the reaction. Pedagogically, this experiment serves as an illustration of kinetics and mechanism as students can visually observe the reaction progress as it changes from yellow to orange-red.
As part of Muskingum’s laboratory-intensive mission, the incorporation of instrumentation and data-collection technology has been at the forefront of the department curriculum for the past 15 years. Today, the chemistry department frequently uses instrumentation and probeware, including the Vernier Emissions Spectrometer, Vernier Mini GC Plus, pH sensors, conductivity probes, and temperature probes, in a variety of lab experiments. The use of these technologies helps to build students’ proficiency using instrumentation while providing them with hands-on experience that will better prepare them for careers in the chemistry field.