Vitamin B2, also known as Riboflavin, is a vital component of the cofactors that support all flavoproteins. The highest concentrations in the body are found in the liver, kidneys, and heart. This vitamin is very important to the heart because it plays a key role in allowing aerobic (oxygen-based) energy production to occur. A great deal of our knowledge about these metabolic and aerobic energy-producing processes is because of the fact that Riboflavin is a highly fluorescent molecule, and it can be investigated using fluorescence spectroscopy.
We can use the SpectroVis Plus spectrophotometer’s fluorescence function to observe Riboflavin in energy drinks. This same technique can be used to investigate the concentration of vitamin B2 in vitamin tablets, foods, and other beverages.
Fluorescence is the emission of light by a compound after it has absorbed a particular wavelength of light. Under most circumstances, the emission of light will occur at a longer wavelength than the light used to excite it. The SpectroVis Plus has two excitation wavelengths, one at 405 nm and 500 nm.
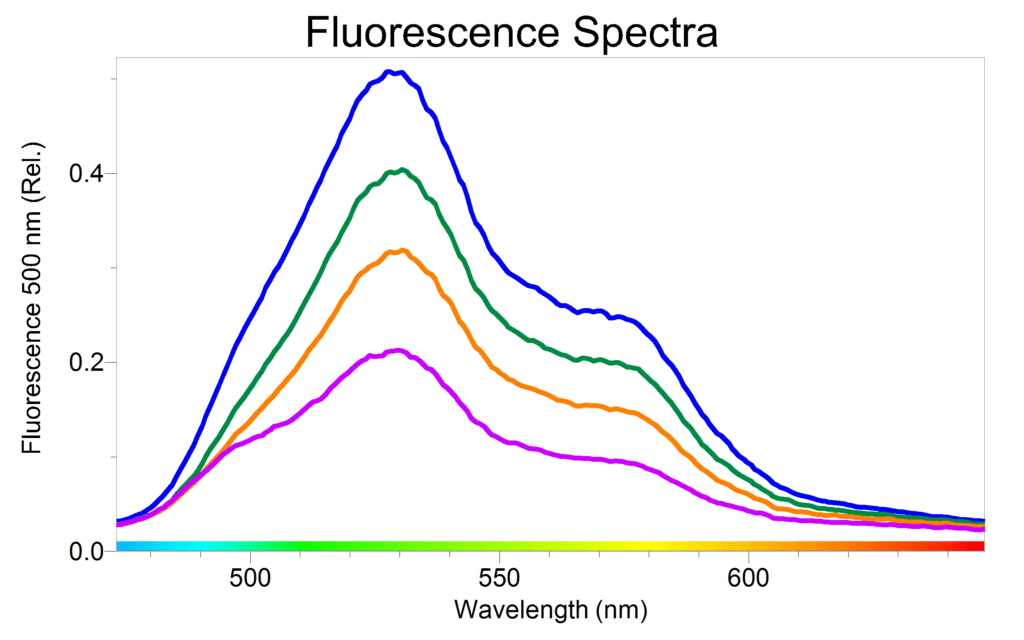
To analyze the concentration of vitamin B2 in an energy drink, such as Rockstar Sugar-free, transfer a small sample of beverage into a cuvette. Make sure that all the bubbles from the carbonation are gone or are out of the pathlength of the white light. Set the SpectroVis Plus to Fluorescence mode with excitation at 500 nm. Collect a spectrum. You should observe at least one peak, with its maximum at 550 nm. If a narrow peak is observed at 500 nm, it is merely scatter from the excitation source. The emission spectrum will contain the emission from Riboflavin.
To verify that the peak is Riboflavin, have your students prepare a standard solution that contains a known source of the compound. It is best to purchase Riboflavin from a chemical company for the most accurate and quantitative results. You can also purchase vitamin B2 tablets at the supermarket and get nice, qualitative results. Dissolve a known mass of Riboflavin in 1% acetic acid in water (diluted vinegar or sparkling water can also work as a solvent). If you are extracting the compound from a vitamin tablet, it is recommended that you filter the solution before use. Dilute this standard solution appropriately to a fluorescence value of approximately 0.5 at its peak wavelength above 510 nm. You should see a peak that looks similar to the one from the energy drink. Keep in mind that some energy drinks may not have a high enough concentration of vitamin B2 to be seen in a fluorescence spectrum. Rockstar Sugar-free works nicely and offers a great starting point to see if other beverages that are relatively translucent have vitamin B2 present.
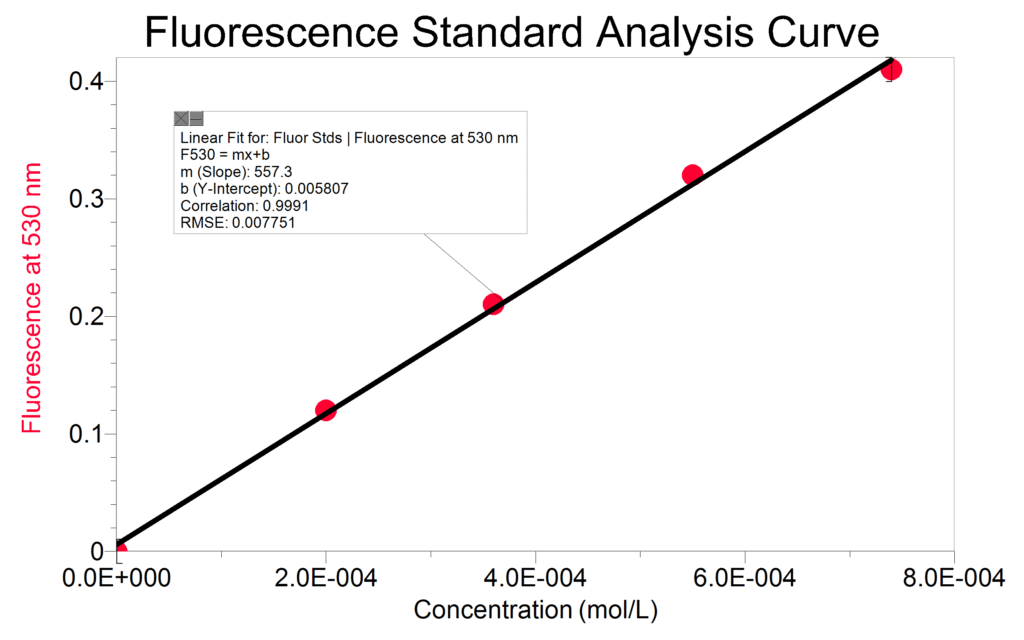
Quantifying Riboflavin with Fluorescence
Due to the high fluorescence quantum yield of Riboflavin, it is also interesting to have your students perform a calibration curve with a similar set of standards using the fluorescence function of SpectroVis Plus. Because Riboflavin peaks at 440 nm, it is appropriate to use either excitation wavelength 405 nm or 500 nm. The data shown was obtained with excitation at 500nm. Excitation at 500 nm exhibits an emission peak at 550 nm. Since the absorbance is so low at this wavelength, the standards should not need to be diluted. Be aware that a calibration curve of the fluorescence of this compound may begin to curve because of self-absorbing; use only the linear region of the calibration curve for analysis.
Monitor the fluorescence value of your energy drink at 550 nm and compare this to your standard calibration curve. At low enough concentrations, the relationship between fluorescence and concentration reduces to a Beer’s law-type analysis. Just keep in mind, that all comparisons this way will be relative and not absolute.
Use this idea as a great way to incorporate fluorescence spectroscopy into your chemistry and biochemistry curriculum; it works particularly well as an inquiry-based experiment.