In an iodine redox titration, starch, which turns blue-black in the presence of iodine, is typically used as the indicator. However, this method can be challenging for students because of the low solubility of iodine in water and the pH dependence of the reaction (which proceeds quantitatively in neutral or slightly acidic solutions). Did you know that there are a couple of variations on this experiment that can create a more successful experience for your students?
The first option, written up by Allan Pinhas and published in the Journal of Chemical Education, presents the use of povidone iodine, also known as betadine, as the stable source of iodine for the redox titration with thiosulfate. The use of povidone iodine is appealing because the solution is slightly acidic and the reddish-brown to clear transition works well as a visual indicator, making it easier for students to identify the equivalence point.
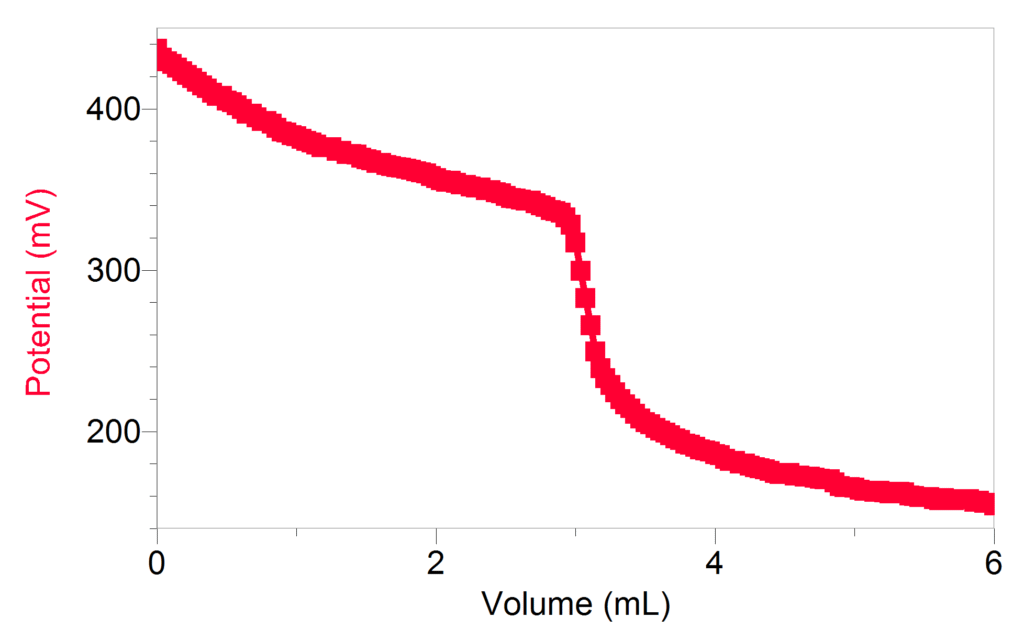
As a second variation, we built on Pinhas’ innovative work and used an ORP (oxidation-reduction potential) Sensor to analyze the reaction, eliminating the reliance on the visual indicator for identifying the equivalence point. Using an ORP Sensor is analogous to using a pH Sensor for an acid-base titration, but the graph displays potential vs. volume rather than pH vs. volume. In the sample data shown, approximately 5 g of povidone iodine solution was dissolved in 25 mL of water. The titration with a 0.1 M sodium thiosulfate solution was monitored using a Vernier ORP Sensor and a Drop Counter. The equivalence point indicates the solution is 0.77% iodine, supporting the 1% iodine claim on the label.
Details for preparing the solutions are available through the Journal of Chemical Education1. For questions about the ORP Sensor or the Drop Counter, email chemistry@vernier.com
1Allan R. Pinhas, “A Redox Titration for a General-Organic-Biochemistry (GOB) Course Using Povidone Iodine,” J. Chem. Educ., 87 (9), 2010, pp. 985–986.
