
Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM

If you’ve ever tried to explain acids and bases and gotten blank stares, it might be time to bring in the hot sauce. I was inspired by this year’s National Chemistry Week theme, “The Hidden Life of Spices,” to cook up a new Vernier activity, which I also shared during a recent webinar.
In this hands-on investigation, students explore the chemical properties of sauces they bring in from home by measuring pH, conductivity, and CO₂ production from a baking soda reaction. Along the way, they see how ingredients like vinegar, salt, oil, sugar, and spices influence these properties—and how culture and chemistry intersect through food.
Making Chemistry Personal with Food
One of the things I like most about this activity is how flexible it is. Every classroom will look a little different depending on what students bring in. A student might share a family hot sauce, a regional dressing, or a homemade spice blend. That variety makes the data more interesting and the learning more personal. It also opens up conversations about the science behind traditional cooking and preservation methods across cultures.
As a teacher, you don’t need every detail mapped out ahead of time—this kind of open-ended activity works best when you let curiosity drive it. What happens if a sauce behaves differently than expected? Why? That’s where engagement—and real sensemaking—happens.

Investigation Overview
This simple, open-ended lab helps students identify relationships among acidity, ionic strength, and reaction behavior in everyday sauces or condiments. Students bring in a favorite sauce from home or choose from a classroom set you provide, then they explore three key questions.
- How acidic is it? (pH)
- How many ions does it contain? (conductivity)
- How much CO₂ does it produce when reacted with baking soda? (acid–base reaction)
These questions naturally connect to core chemistry concepts and NGSS standards:
- Acid–base chemistry
- Ionic compounds and solution chemistry
- Experimental design and data interpretation
- Cultural identity and scientific inquiry
NGSS Connections
- HS-PS1-2: Construct explanations for chemical reactions based on evidence.
- HS-PS1-5: Apply scientific principles to design and conduct an investigation on chemical properties.
Materials Needed
- Go Direct Tris-Compatible Flat pH Sensor
- Go Direct Conductivity Probe
- Go Direct CO₂ Gas Sensor
- Device running Vernier Graphical Analysis®
- Beakers, reaction containers, spatulas
- Deionized water and Baking soda (NaHCO₃)
- Student- or instructor-provided sauces (e.g., hot sauce, mole, sriracha, fish sauce, ketchup, lemon juice, marinara, vinaigrettes)
Procedure Overview
Ideally, you’ll want to look at sauces that highlight a range of properties—something acidic (vinegar- or lemon–based), salty (soy or fish sauce), and thick or oily (hot sauce or vinaigrette). Vinegar and olive oil work well as reference samples.
Before testing, have students predict which sauces will be most acidic or most conductive and share their reasoning. This activity doesn’t need to be perfect or polished—it’s exploratory, not prescriptive—and that flexibility is what makes it so engaging.
Part 1: pH Measurement
- Dilute the sauce 1:2 with deionized water.
- Measure pH using the pH Sensor.
- Analyze and discuss results.
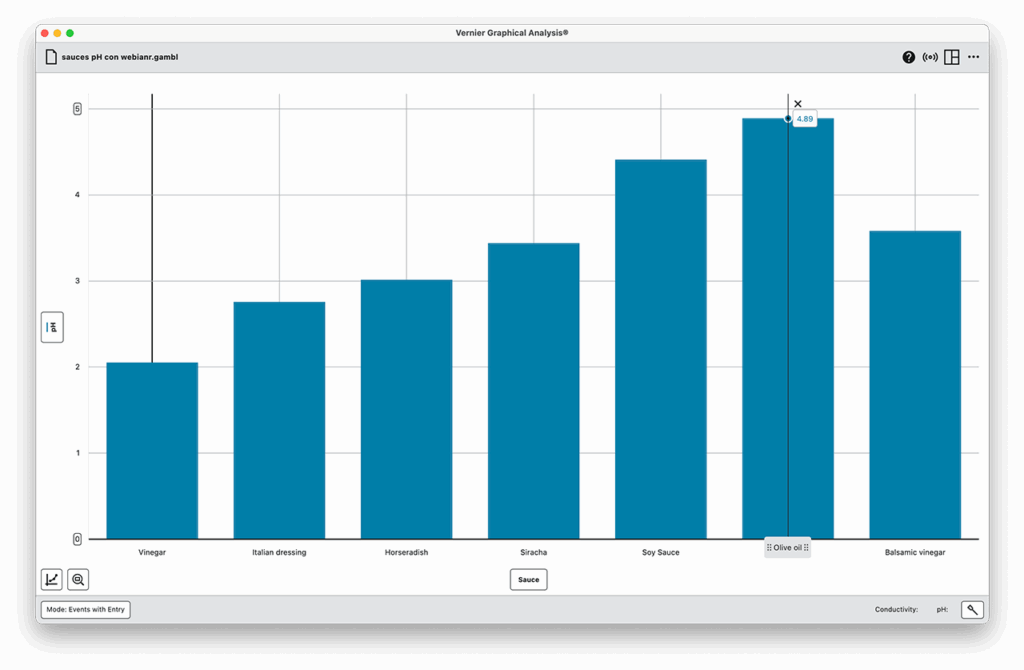
Part 2: Conductivity
- Use the same diluted sample.
- Measure conductivity in µS/cm with the Conductivity Probe.
- Analyze and discuss results.
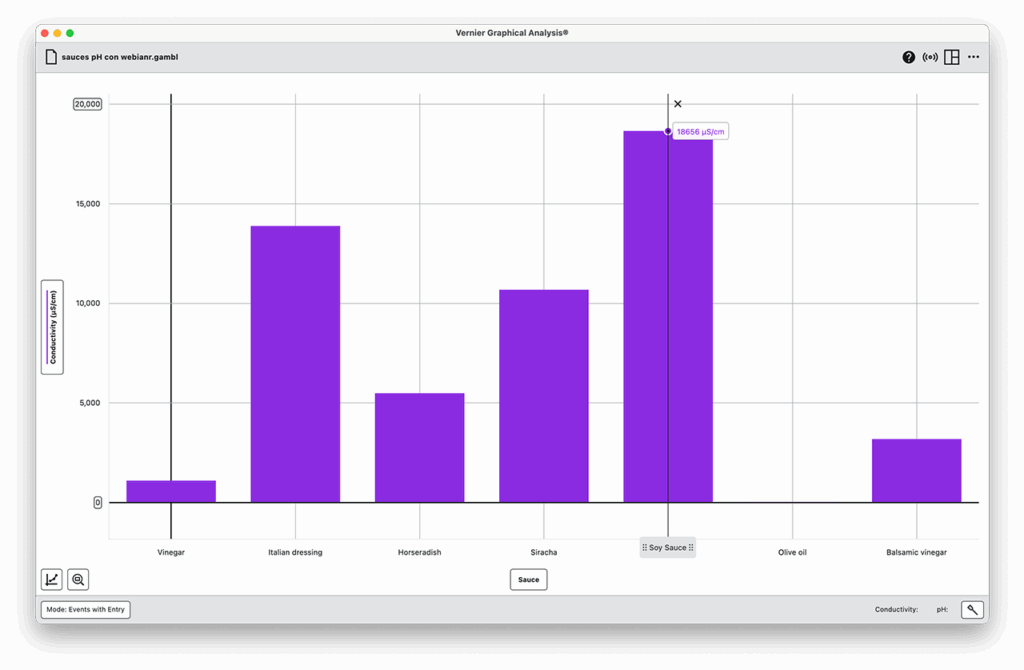
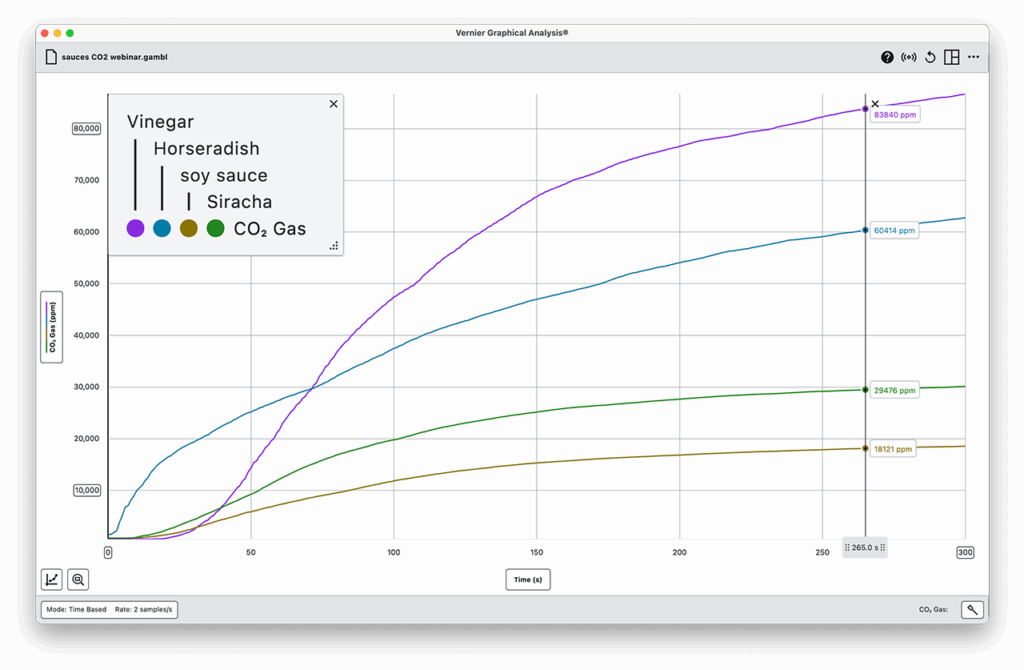
Part 3: CO₂ Reaction
- Add 10 mL of sauce to a sealed reaction container.
- Add 0.5 g baking soda.
- Immediately seal and measure CO₂ production using the CO₂ Gas Sensor.
- Record peak CO₂ level (ppm). Analyze and discuss results.
What Students Learn
I’ve always found that the quickest way to get students curious about chemistry is through food. Sauces are complex mixtures—they contain acids, salts, sugars, and oils—and each ingredient behaves differently in a reaction.
Here’s what students should take away from this investigation, as well as some interesting observations we made during some early trials:
Key Takeaways
- Acidity influences chemical reactivity, but not all low-pH sauces react equally.
- Conductivity reflects ionic content from salt, acids, and fermented ingredients.
- CO₂ production is influenced by pH, ion availability, and physical properties (like oil or thickeners).
Real Observations from the Lab
- Fish sauce had extremely high conductivity (~31,000 µS/cm) due to its salt and protein content but produced low CO₂, likely because of buffering and moderate pH.
- Horseradish and Italian dressing produced large amounts of CO₂ despite lower acidity, showing that ion mobility and texture play important roles.
- Sugar- and oil-rich sauces (like sweet chili or vinaigrettes) showed reduced CO₂ release, likely due to diffusion barriers.
Making Real-World Connections
I’m sure you can imagine a student asking, “Okay, now that we know the pH of Italian dressing, why does that matter?” and that’s a great question. Acids and ions don’t just change flavor—they change structure. Acids affect the texture in foods like pickles and bread, helping with leavening and protein denaturation. Salts drive ion concentration, influencing preservation and fermentation.
For example, fish sauce nearly maxed out the conductivity sensor—about twice that of soy sauce. That extreme ion content explains why it’s such an effective preservative and a key ingredient in many fermented dishes.
| Use Case | Look for Acidity (pH) | Look for Ion Content (Conductivity) |
| Baking with baking soda | ✓ Required for leavening | ✕ Not relevant |
| Brining meats | ✕ Not important | ✓ Critical for effectiveness |
| Pickling vegetables | ✓ Key for preservation | ✓ Helps flavor, but not essential |
| Making soup stock | ✕ | ✓ For flavor balance and conductivity |
| Fermentation (kimchi) | ✓ Develops over time | ✓ Salt ions crucial for microbial control |
| Ceviche (acid cooking) | ✓ Essential to denature proteins | ✕ |
How can this type of analysis help in food science or culinary chemistry?
The takeaway: Acidity and ion concentration each tell part of the story—and both shape the chemistry of food, from how it feels to how long it lasts.
Why This Investigation Works
This investigation checks all the boxes for meaningful classroom engagement:
- Safe, edible, and low waste, supporting green chemistry principles.
- Adaptable—intro classes can focus on pH, while advanced students can explore reaction kinetics.
- Authentic—students collect and interpret their own data, not just watch a demonstration.
Most importantly, it’s fun. Chemistry doesn’t have to feel abstract when students can see it, smell it, taste it, measure it, and connect it to something from their own kitchens.
Try It Out in Your Classroom
You can watch the Chemistry of Sauces webinar on demand for a walkthrough of the setup and sample data.
Looking for more food chemistry to explore the hidden life of spices? Check out our food chemistry solutions or explore other sustainable experiments from our green chemistry solutions.
Let us know if you give this activity a try in your classroom and share with us on social. Questions? Reach out to chemistry@vernier.com, call 888‑837‑6437, or drop us a line in the live chat.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.






