Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM

When I was teaching in the classroom, chemical equilibrium was not only a difficult topic for students to understand, but it was also a tricky topic to teach. Students can’t see molecules shifting between reactants and products, and the concept of a “dynamic” balance can feel abstract. That’s why I’m always looking for ways to make equilibrium more tangible, visually engaging, and fun.
Recently, I was inspired by a lab from Beyond Benign (and some cool videos I saw on TikTok) to create a colorful, safe, and sustainable food chemistry experiment using butterfly pea tea to model acid-base equilibrium shifts. This simple setup brings concepts like Le Châtelier’s Principle and pKa to life, while letting students collect real-time data using a Go Direct® pH Sensor and a Vernier Go Direct SpectroVis® Plus Spectrophotometer.
This experiment combines a striking visual phenomenon, hands-on pH and absorbance measurements, and a strong green chemistry connection to create a memorable learning experience.
Why This Lab Works for Teaching Equilibrium
In my chemistry classes, I often saw the same kinds of misconceptions come up with from students:
- “Equilibrium means nothing is happening.”
- “Once the color change stops, the reaction’s over.”
- “Adding something just makes it more acidic or basic—it doesn’t shift anything.”
The truth is that equilibrium is dynamic. Even when a solution looks “stable,” molecular reactions are still happening. A stress, like changing pH, shifts the system. And in this case, that molecular-level shift is visible through color.
Butterfly pea tea contains anthocyanins, natural plant pigments whose structure changes with pH. These changes alter how the molecules interact with light, creating distinct colors for acidic, neutral, and basic solutions. Plus, this experiment follows the principles of green chemistry: no heavy metals, no synthetic dyes, just a visually engaging, student-safe chemical reaction.
Phenomenon First: Why Did My Tea Turn Purple?
To introduce this experiment, start with sharing a quick demo or video of the color‑change phenomenon. Or, if time allows, share a longer video that gives more context and explanation to the origins of the tea.
Give students a moment to react, then prompt some initial thoughts by asking: “Why did that color change happen? What’s changing in the tea?” In student pairs or as a classroom discussion, you can brainstorm how pH might relate to color and discuss other examples they may have noticed about food or drinks changing colors with additives.
Experiment Setup

For the Class
- Prepared butterfly pea tea (~50 mL per group)
- Additives, including: distilled water, lemon juice, vinegar, baking soda solution, Sprite®, and Alka‑Seltzer® solution
For Each Group of Students
- Vernier Graphical Analysis® app and Vernier Spectral Analysis® app running on a Chromebook™, computer, or mobile device
- Go Direct pH Sensor (or Go Direct Tris‑Compatible Flat pH Sensor)
- Go Direct SpectroVis Plus Spectrophotometer
- 6 test tubes with rack
- 7 cuvettes with rack (1 for calibration and 6 for the test solutions)
- 10 mL graduated cylinder, transfer pipettes, small scoop (~0.2 g), and safety goggles
Investigating Color Change, pH, and Absorbance
With this lesson, students can see equilibrium shift in real time, measure the change with Vernier tools, and connect those observations to chemical principles they’ll use again and again. This lab unfolds in three main parts, with an optional fourth for advanced exploration.
Part 1: Prepare Solutions and Observe Color Changes
Students prepare six tea samples with different additives that create acidic, basic, and neutral conditions. They record the immediate visual changes, noting how quickly the reaction happens.
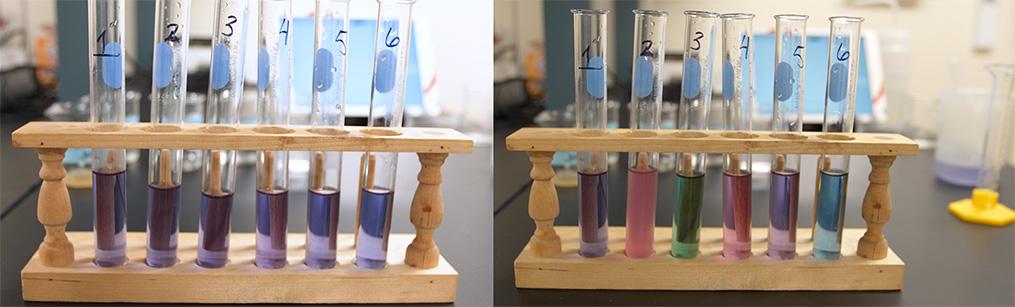
Ask students to predict what would happen if they reversed the process (acid to base, base to acid).
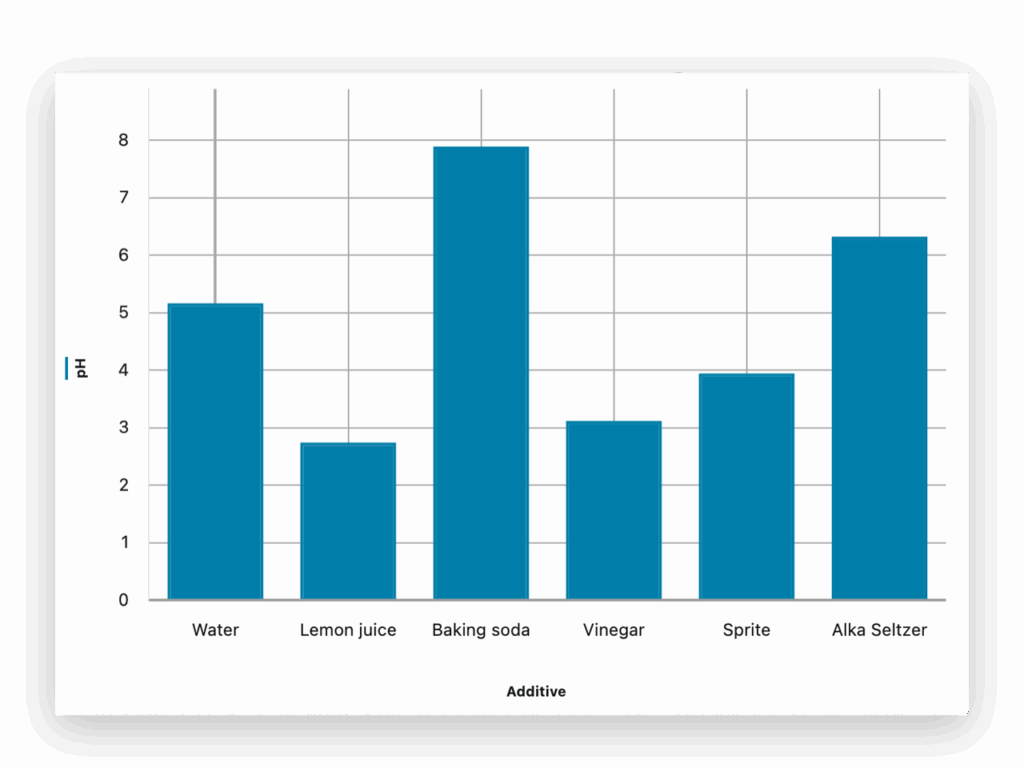
Part 2: Measure pH
Students quantify the pH of each sample, linking numerical data to observed colors. This helps them connect qualitative and quantitative evidence and evaluate their predictions from part 1.
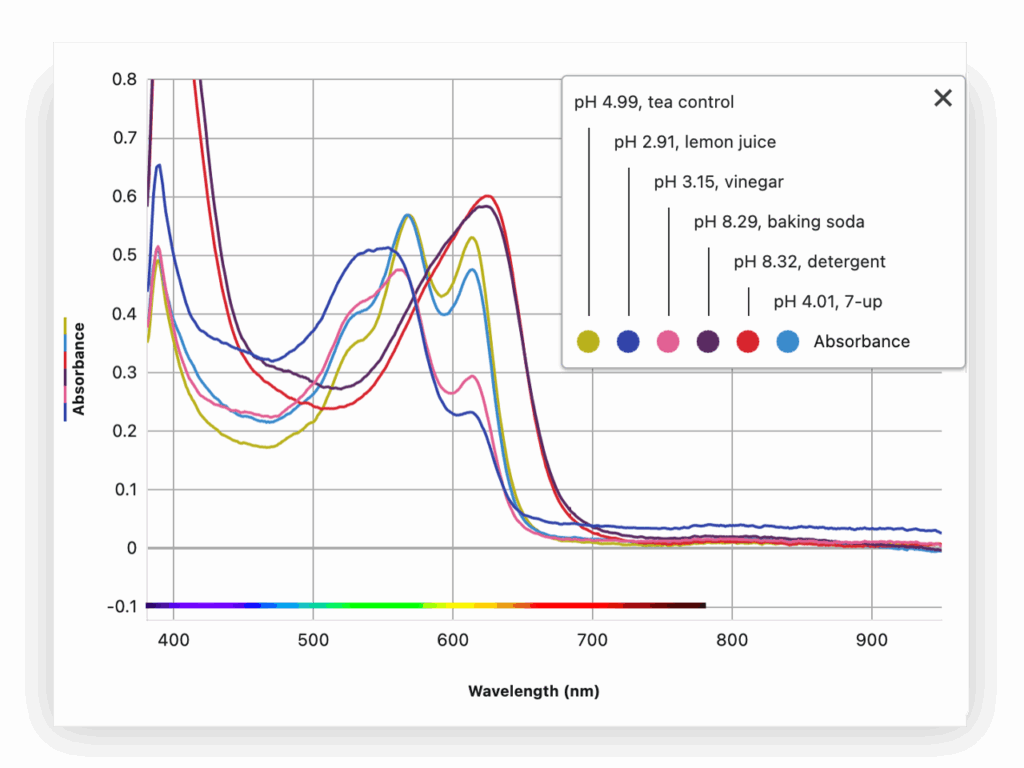
Part 3: Measure Absorbance Spectrum
Using the spectrophotometer, students collect absorbance spectra for each sample and identify how peak wavelengths shift with pH. They relate these changes to molecular structure and perceived color.
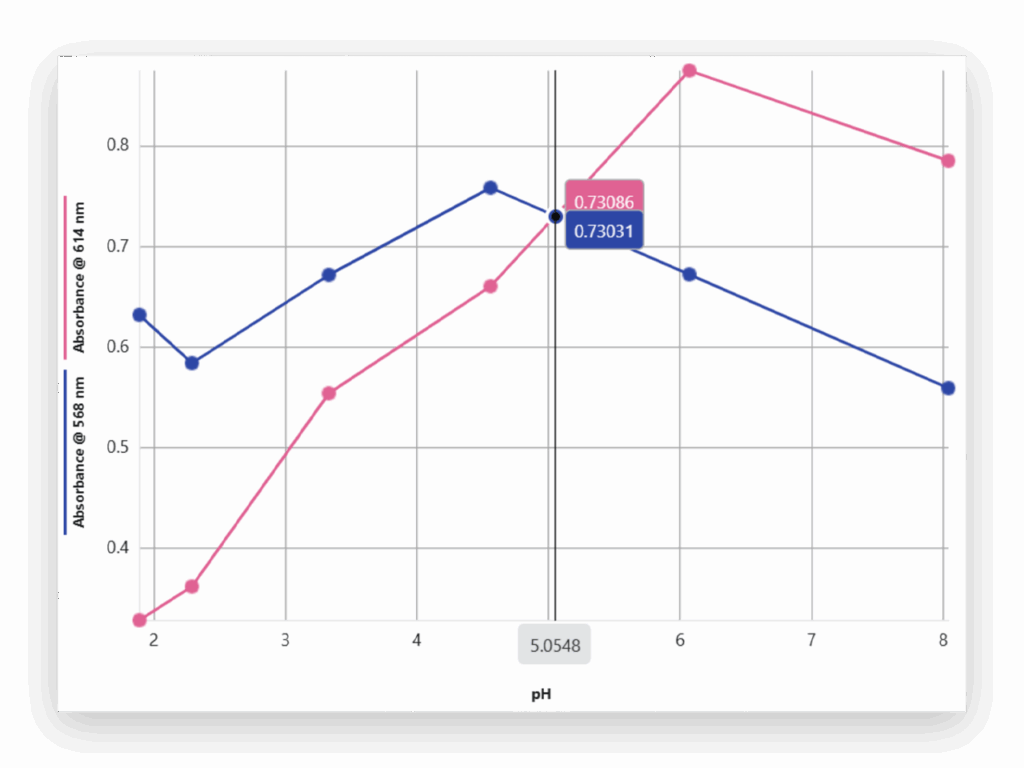
Part 4: Multiwavelength Absorbance vs. pH (Optional)
For advanced or AP-level classes, students measure absorbance at two key wavelengths and plot absorbance vs. pH to estimate the pKa of the tea’s anthocyanin pigment.
Green Chemistry Benefits and NGSS Alignment
Traditional equilibrium demos often use synthetic indicators or metal complexes like iron(III) thiocyanate. Using tea instead is a non-toxic alternative that reduces waste, is safe to handle, and easier to dispose of.
This experiment aligns with NGSS performance expectations for structure and properties of matter, chemical reactions, and refining chemical system designs.
Try It in Your Classroom
- Download the full student and instructor experiment: Free 2025 Back-to-School Sampler
- Watch the full webinar recording: A Perfect Brew
- Explore our Green Chemistry resources
I love this experiment because it’s playful and rigorous. It captures attention, piques curiosity, and drives home a concept that can otherwise feel frustratingly abstract. Plus, there’s just something magical about watching that beautiful color shift bloom in real time.
If you try it, let us know how your students react!
Have you developed or adapted a green chemistry lab experiment using Vernier technology? We’ve partnered with Beyond Benign and the Green Chemistry Teaching and Learning Community (GCTLC) to build a free collection of educator-written, sustainable experiments and resources for K–12 and higher education instruction. All submissions will be peer‑reviewed, and original work not published elsewhere may be assigned DOIs upon request. This call is open through November 30, 2025.
We want to hear about your innovative ways of teaching chemistry with Vernier! Let us know at blog@vernier.com or share with us on social. Questions? Reach out to chemistry@vernier.com, call 888‑837‑6437, or drop us a line in the live chat.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.