Experiments
Here are experiments our science specialists have selected to support this IB* topic.
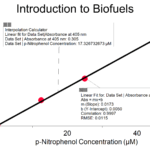
Introduction to Biofuels: Enzyme Action
Experiment #7 from Investigating Biology through Inquiry
In this Preliminary Activity, you will use a spectrophotometer to determine p-nitrophenol concentration. You will first produce a p-nitrophenol containing sample with the reaction. Next, you will determine the absorbance values for a set of p-nitrophenol solutions with known concentrations in order to create a standard curve. When a graph of absorbance vs. concentration is plotted for the standard solutions, a direct relationship should result. The direct relationship between absorbance and concentration for a solution is known as Beer’s law. You will then determine the p-nitrophenol concentration of your prepared sample by measuring its absorbance and then finding the corresponding concentration on the standard curve. Because the relationship is linear, you could also calculate the
p-nitrophenol concentration using the formula for the standard curve. You will also determine the initial rate of your p-nitrophenol producing reaction.
After completing the Preliminary Activity, you will first use reference sources to find out more about biofuels and the cellobiase catalyzed reaction that produces p-nitrophenol before you choose and investigate a researchable question. Some topics to consider in your reference search are:
- catalyst
- enzyme
- biofuel
- ethanol
- cellulose
- cellobiose
- cellobiase
- substrate
- p-nitrophenol
- Beer’s law
- Educational Standard
- International Baccalaureate (IB) 2025
- Subject
- Biology
- Theme
- B Form and function
- Level of organization
- 1 Molecules
- Topic
- B1.1 Carbohydrates and lipids
- Section
- Standard level and higher level
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
