Experiments
Here are experiments our science specialists have selected to support this IB* topic.
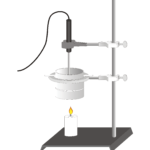
Fossil Fuels
Experiment #26 from Investigating Environmental Science through Inquiry
In the Preliminary Activity, you will determine the heat of combustion of paraffin wax (in kJ/g). You will first use the energy from burning paraffin wax to heat a known quantity of water. By monitoring the temperature of the water, you can find the amount of heat transferred to it (in kJ), using the formula
where q is heat, Cp is the specific heat capacity of water, m is the mass of water, and Δt is the change in temperature of the water. Finally, the amount of fuel burned will be taken into account by calculating the heat per gram of paraffin wax consumed in the combustion.
After completing the Preliminary Activity, you will first use reference sources to find out more about fossil fuel energy before you choose and investigate a researchable question.
- Educational Standard
- International Baccalaureate (IB) 2025
- Subject
- Biology
- Theme
- C Interactions and interdependence
- Level of organization
- 4 Ecosystems
- Topic
- C4.2 Transfers of energy and matter
- Section
- Standard level and higher level
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
