Introduction
The decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is stable for a long period of time. The decomposition takes place according to the reaction below.
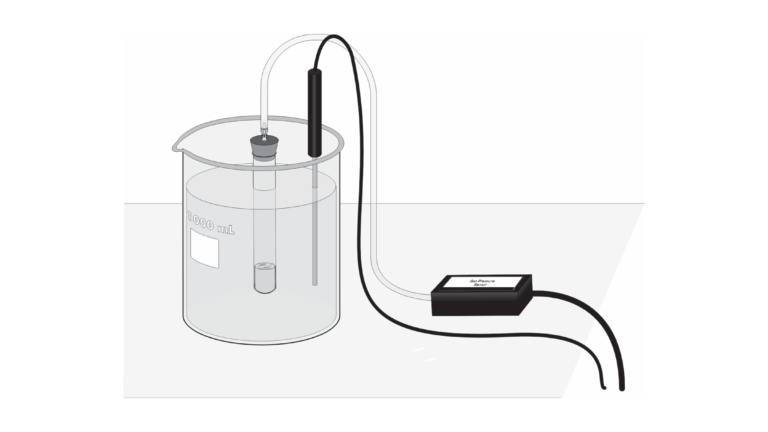
A number of catalysts can be used to speed up this reaction, including potassium iodide, manganese (IV) oxide, and the enzyme catalase. If you conduct the catalyzed decomposition of hydrogen peroxide in a closed vessel, you will be able to determine the reaction rate as a function of the pressure increase in the vessel that is caused by the production of oxygen gas. If you vary the initial molar concentration of the H2O2 solution, the rate law for the reaction can also be determined. Finally, by conducting the reaction at different temperatures, the activation energy, Ea, can be calculated.
Objectives
In this experiment, you will
- Conduct the catalyzed decomposition of hydrogen peroxide under various conditions.
- Calculate the rate constant for the reaction.
- Determine the rate law expression for the reaction.
- Calculate the activation energy for the reaction.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Reactivity 2.2.1—The rate of reaction is expressed as the change in concentration of a particular reactant/product per unit time.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #12 of Advanced Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.





