Conductimetric Titrations
Experiment #18 from Investigating Chemistry through Inquiry
- Subject
- Chemistry
Introduction
There are a number of ways to investigate a chemical reaction. Measuring temperature change, pressure change, pH change, and/or conductivity change are some of the ways. A titration is a process used to determine the volume of a solution needed to react with a given amount of another substance. You have probably done pH titrations in other experiments; now you will be performing conductivity titrations. In the Preliminary Activity, you will titrate a solution of the strong acid hydrochloric acid, HCl, with a solution of the strong base sodium hydroxide, NaOH. The concentration of the NaOH solution is given, and you will determine the unknown concentration of the HCl. Hydrogen ions from the HCl react with hydroxide ions from the NaOH in a one-to-one ratio to produce water in the overall reaction:
Objectives
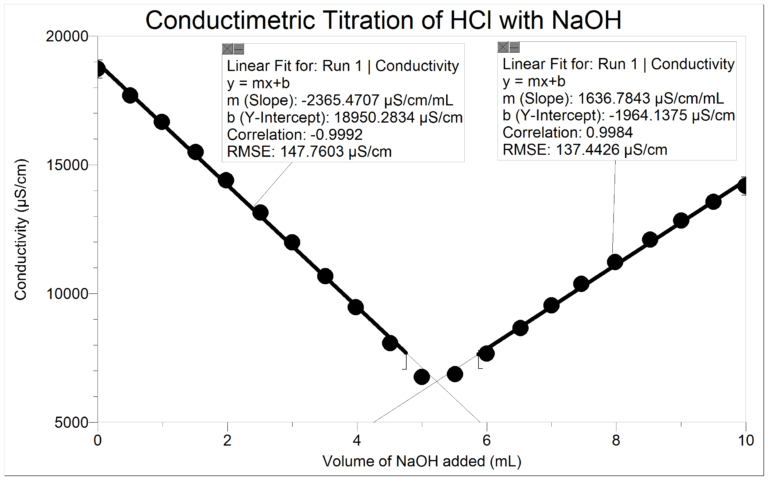
You will determine the volume of NaOH titrant used at the equivalence point using the intersection of the linear fits of the two branches of the resulting curve. The volume of NaOH titrant used at the equivalence point will be used to determine the molarity of the HCl.
After completing the Preliminary Activity, you will first use reference sources to find out more about conductimetric titrations before you choose and investigate a researchable question involving conductimetric titrations.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #18 of Investigating Chemistry through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

