Baking Soda and Vinegar Investigations
Experiment #2 from Investigating Chemistry through Inquiry
- Subject
- Chemistry
Introduction
Sodium bicarbonate is a widely used compound with the formula NaHCO3. It is also known as baking soda, bicarbonate of soda, cooking soda, and sodium hydrogen carbonate. Vinegar is a dilute solution of acetic acid, HC2H3O2, made by the fermentation of wine or some other solution containing ethanol.
Baking soda reacts with acetic acid in vinegar to produce carbon dioxide gas, water, and an aqueous solution of sodium acetate according to the equation:
Objectives
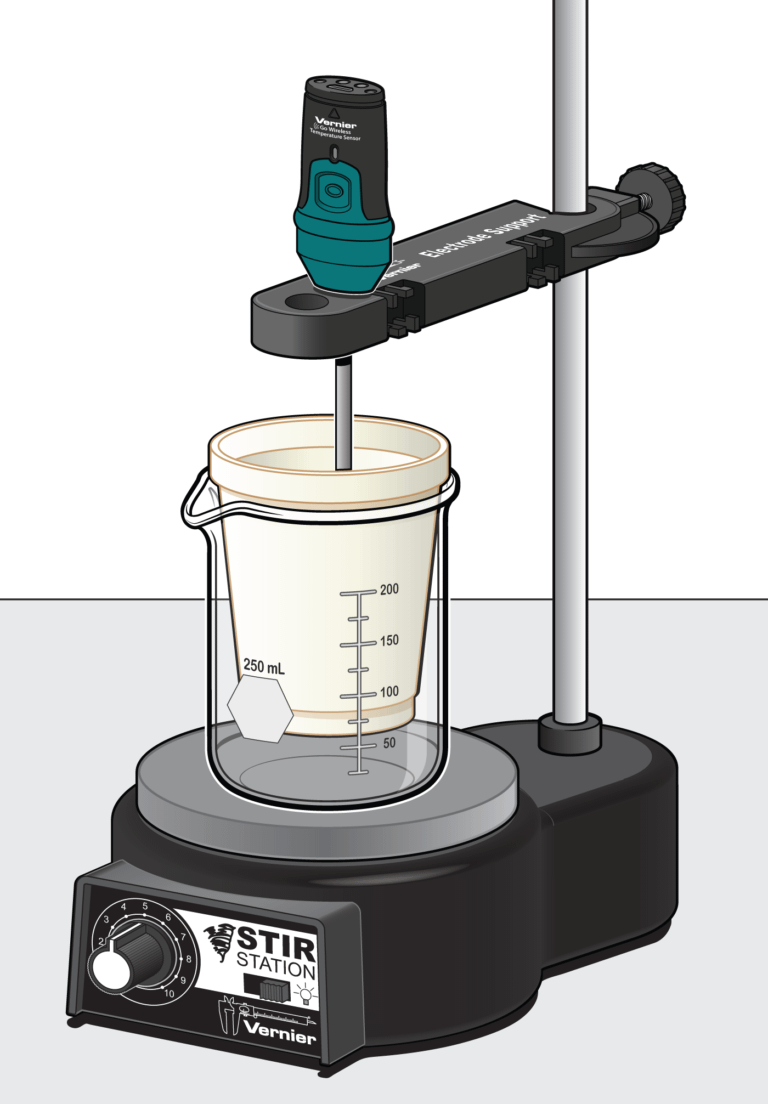
In the Preliminary Activity, you will gain experience using a Temperature Probe as you determine the temperature change when 2.00 g of baking soda is added to and reacts with 50.0 mL of vinegar.
After completing the Preliminary Activity, you will first use reference sources to find out more about baking soda and vinegar before you choose and investigate a researchable question dealing with the properties of baking soda and/or vinegar or the reaction between them.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #2 of Investigating Chemistry through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


