Investigating the Energy Content of Foods
Experiment #6 from Investigating Chemistry through Inquiry
- Subject
- Chemistry
Introduction
Food supplies energy for all animals—without it we could not live. The quantity of energy stored in food is of great interest to humans. The energy your body needs for running, talking, and thinking comes from the foods you eat. Not all foods contain the same amount of energy, nor are all foods equally nutritious for you. An average person should consume a minimum of 2,000 kilocalories per day. That is equivalent to 8,360 kilojoules. Calories and joules are both units of energy. We will use joules in this experiment since it is the accepted SI metric standard.
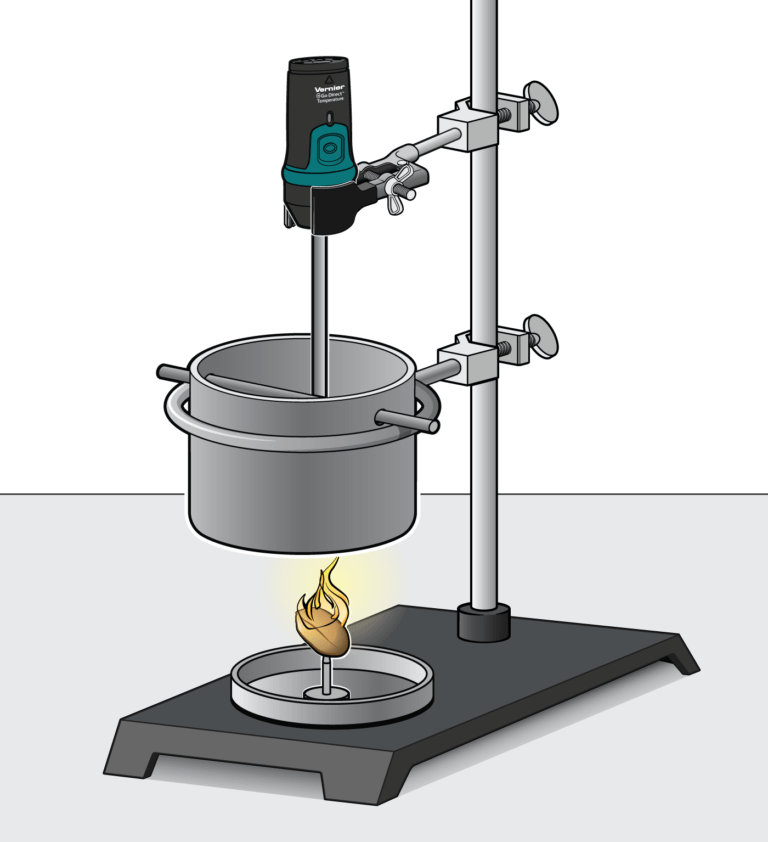
You can determine energy content of food by burning a portion of it and capturing the heat released to a known amount of water. This technique is called calorimetry. The energy content of the food is the amount of heat produced by the combustion of 1 gram of the food, and is measured in kilojoules per gram (kJ/g).
Objectives
In the Preliminary Activity, you will determine the energy content of a peanut. You will first use the energy from a burning peanut to heat a known quantity of water. By monitoring the temperature of the water, you can find the amount of heat transferred to it (in kJ), using the formula
where q is heat, Cp is the specific heat capacity of water, m is the mass of water, and Δt is the change in temperature of the water. Finally, the amount of peanut burned will be taken into account by calculating the heat per gram of peanut consumed in the combustion.
After completing the Preliminary Activity, you will first use reference sources to find out more about calorimetry, food, and food energy sources before you choose and investigate a researchable question dealing with the energy content of food.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #6 of Investigating Chemistry through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


