Investigating the Energy Content of Fuels
Experiment #7 from Investigating Chemistry through Inquiry
- Subject
- Chemistry
Introduction
Hydrocarbons are compounds containing only hydrogen and carbon atoms. Many common fuels such as gasoline, diesel fuel, heating oil, aviation fuel, and natural gas are essentially mixtures of hydrocarbons. Paraffin wax, used to make many candles, is a mixture of hydrocarbons with the representative formula C25H52.
Ethanol, a substituted hydrocarbon with the formula CH3CH2OH, is used as a gasoline additive (gasohol) and as a gasoline substitute. Ethanol is an alcohol—a hydrocarbon derivative in which one or more hydrogen atoms has been replaced by a hydroxyl group (–OH). 1-propanol (CH3CH2CH2OH), 2-propanol (CH3CH(OH)CH3), and 1-butanol (CH3CH2 CH2CH2OH) are other common alcohols.
Objectives
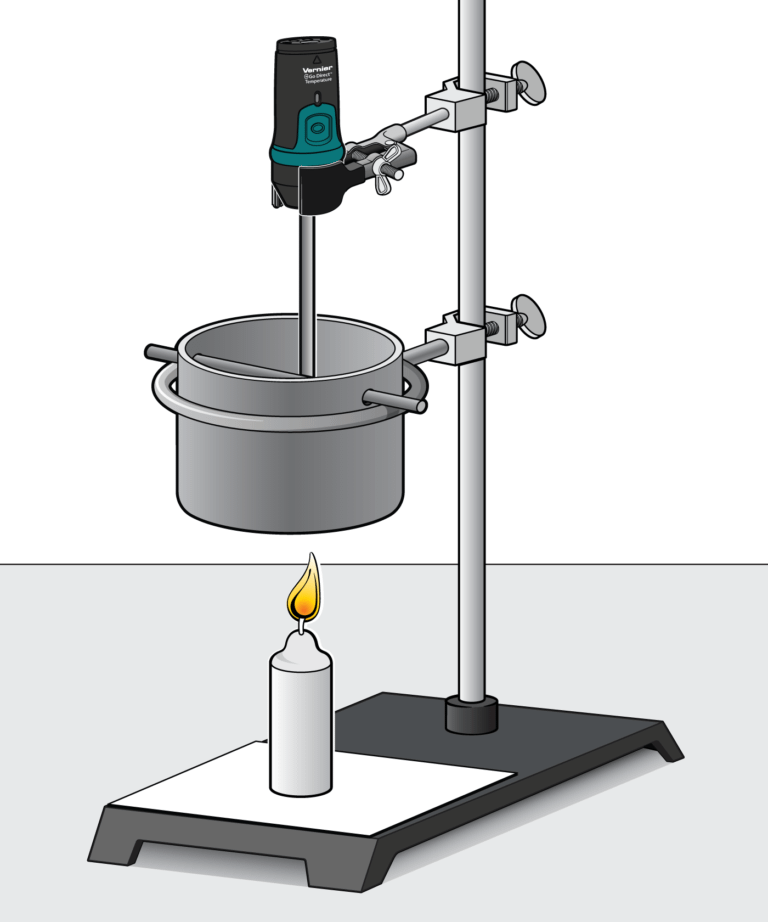
In the Preliminary Activity, you will determine the heat of combustion of paraffin wax (in kJ/g). You will first use the energy from burning paraffin wax to heat a known quantity of water. By monitoring the temperature of the water, you can find the amount of heat transferred to it (in kJ), using the formula
where q is heat, Cp is the specific heat capacity of water, m is the mass of water, and Δt is the change in temperature of the water. Finally, the amount of fuel burned will be taken into account by calculating the heat per gram of paraffin wax consumed in the combustion.
After completing the Preliminary Activity, you will first use reference sources to find out more about calorimetry and fuels before you choose and investigate a researchable question.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #7 of Investigating Chemistry through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


