Introduction
If you were to view the planet Earth from space, you would see that most of its surface is covered by water. Most of this is ocean water which cannot be consumed by humans for the purpose of hydration. Why not? The reason is that ocean water contains a large amount of salt, which makes it undrinkable.
Salinity is a measurement of the saltiness or concentration of salt in water. Ocean water contains many different salts, but the most abundant is sodium chloride, also known as table salt. Sodium chloride makes up 86% of all the ions present in ocean water. Other salts that can be found in ocean water at significant levels are calcium chloride and magnesium chloride.
Why is the ocean salty? When the earth was being formed, volcanoes ejected large amounts of lava and chemicals (including salts) into the oceans and atmosphere. Some of these salts dissolved in the water. Rain also washes salts from the land into the ocean. Over time the level of dissolved salts in the oceans increased to the level it is today.
While the average salinity of ocean water is 35 ppt there are several factors that can increase or decrease its salinity. At the polar regions, freezing of ocean water increases the salinity of the surrounding water because very little salt is included in the ice. Evaporation in hot arid regions also increases the salinity. The Dead Sea, located between Israel and Jordan, has a salinity nearly seven times that of most ocean water. At the ocean’s surface, rain, snow, and melting ice are all responsible for decreasing the salinity. As rivers enter the ocean, they carry large volumes of fresh water into the ocean, causing the salinity to decrease.
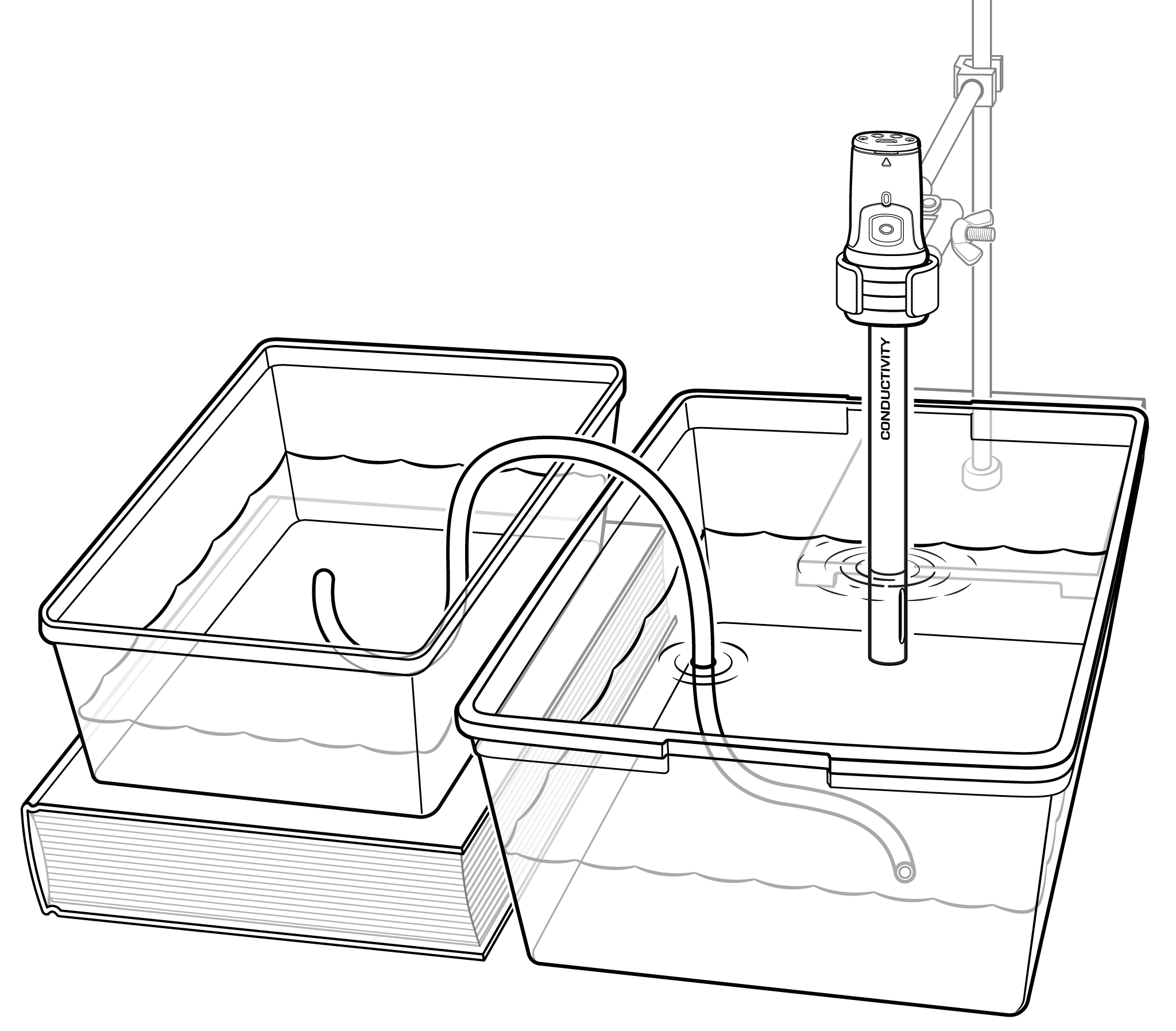
In this experiment, you will use a conductivity probe to measure the salinity of saltwater. In Part I, you will measure the change in ocean salinity due to evaporation. In Part II, you will measure the change in salinity near the mouth of a river as it flows into an ocean.
Objectives
In this experiment, you will
- Measure salinity of a water sample using conductivity probe.
- Determine the effect of evaporation on the salinity of ocean water.
- Determine the salinity change when a river flows into an ocean.
- Calculate salinity changes.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #15 of Earth Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.