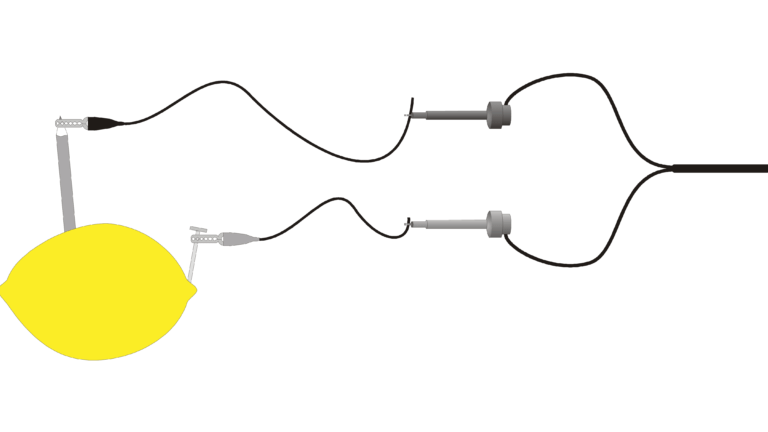
Introduction
“Juice” is a slang term sometimes used for electricity. Batteries are made up of one or more electrochemical cells. Electrochemical cells often consist of two different materials in an electrolytic solution and connected to each other by a wire. In this experiment, you will study some basic principles of electrochemical cells using the juice of a lemon as the electrolyte. You will place small pieces of two different materials into the lemon, and a computer will be used to measure and display the voltages produced.
Objectives
In this experiment, you will
- Build several cells.
- Measure and display cell voltages.
- Discover which combinations produce a voltage.
- Decide which combination makes the “best” battery.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Option 3

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #33 of Physical Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

