
Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM

An Oxidation Reduction Potential (ORP) Sensor measures the real-time activity of oxidizers and reducers in a wide range of aqueous solutions, including natural waters, industrial effluents, and biological samples. Also referred to as a redox measurement, ORP is a potentiometric measurement from a two-electrode system similar to a pH sensor. However, unlike a pH sensor, an ORP sensor measures the ratio of oxidized to reduced forms of all chemical species in solution.
Why Study ORP?
Understanding ORP is crucial for many modern technologies, including fuel cells, batteries, and water treatment systems. By investigating redox reactions, students gain insight into how these technologies work and their significance in society. ORP is relevant to a wide range of applications, and developing a strong understanding of redox reactions is fundamental for exploring more advanced chemistry concepts, such as electrochemistry, electrolysis, and thermochemistry.
Studying ORP not only enhances understanding of real-world industry applications in STEM, but also builds crucial problem-solving and critical thinking skills, including:
- Visualizing conceptual processes
- Performing quantitative analysis
- Connecting theoretical and practical measurements
- Relating data to standards
Applications
Titration and Redox Reactions: Use the ORP Sensor to determine the equivalence point in oxidation-reduction titrations. This hands-on approach helps students learn about chemical reactions, electron transfer, oxidation states, and how substances can act as oxidizing or reducing agents.
Water Quality Monitoring: Investigate the importance of water quality parameters, including ORP, in assessing the health of aquatic ecosystems or drinking water quality. ORP measurements can indicate the presence of pollutants and the overall oxidative or reductive conditions of water.
Biochemical Processes: Explore how ORP affects and is affected by biochemical processes, such as microbial metabolism in natural and engineered environments. Discuss the role of ORP in processes like wastewater treatment and composting.
Environmental Chemistry: Use ORP to discuss the environmental impact of chemicals and pollutants. Students can learn about the oxidative degradation of contaminants and the role of natural reducing agents.
Chemical Kinetics and Equilibrium: Investigate reaction rates and equilibrium. ORP measurements can demonstrate how redox reactions reach equilibrium and how conditions like pH and temperature affect reaction rates.
Food Chemistry: Explore the role of ORP in food chemistry applications, such as preservation, safety, and quality. Discuss how ORP measurements can help monitor and control the oxidation-reduction conditions in food processing.
Electrochemistry: Explore electrochemical cells, electrode potentials, and how ORP relates to the standard electrode potentials. Discuss the Nernst equation and its application in calculating cell potentials.
The Sensor Technology
The sensor is made up of two electrochemical half cells where the reference electrode is generally Ag/AgCl and the measurement electrode is commonly Pt. The potential difference between the two electrodes represents the redox potential of the solution being measured and can be described by the Nernst equation.
E = Eo – 2.3 (RT/nF) x log ([Ox] / [Red])
Where:
- E = total potential developed between the measurement and reference electrodes
- Eo = a voltage specific to the system
- R = gas constant
- T = temperature in K
- n = the number of electrons involved in the equilibrium between the oxidized and reduced species
- F = Faraday constant
- [Ox] = concentration of the oxidized species
- [Red] = concentration of the reduced form of that species
The output of the ORP sensor is relative to the reference electrode. For example, a reading of +100 mV indicates the potential is 100 mV higher than the potential of the reference half cell and suggests an oxidizing environment. Likewise, a –100 mV reading indicates a potential 100 mV lower than the reference half cell and is a reducing environment. In some applications, redox potential may be reported as Eh which is the voltage reading with respect to the Standard Hydrogen Electrode (SHE). By taking into account the offset of the reference electrode used in the ORP sensor, the potential can be converted into Eh readings. Vernier ORP sensors use a Ag/AgCl saturated KCl reference electrode.
Educational Applications
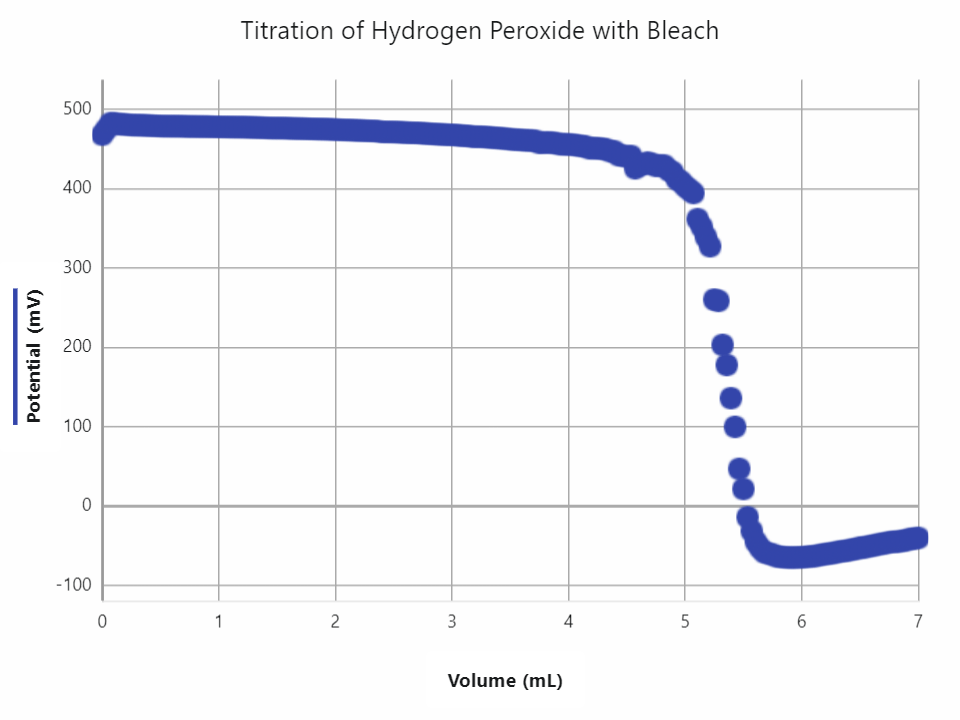
In education, a common application for an ORP sensor is a potentiometric titration. Similar to an acid-base titration, a titrant is added to a sample incrementally until all the sample has reacted and the end-point is reached. One example where students can apply their understanding of redox is by using a Vernier Go Direct® ORP Sensor to determine the concentration of H2O2 by titrating the solution with KMnO4. To correctly calculate the concentration, students must understand the balanced redox reaction between KMnO4 and H2O2.
Another example is using ORP to teach concepts related to energy storage and renewable energy through the investigation of a redox flow battery. Students can measure the ORP of an electrolyte solution before and after charging and discharging cycles. This will help them understand the principles of energy storage in redox flow batteries and to evaluate the efficiency of a redox flow battery system.
Experiments
Check out these additional experiments from Advanced Chemistry with Vernier using an ORP Sensor.
- Potentiometric Titration of Hydrogen Peroxide
- An Oxidation-Reduction Titration: The Reaction of Fe2+ and Ce4+
Explore all our chemistry solutions. Do you have questions about using the Go Direct ORP Sensor? We’re here to help! Reach out to chemistry@vernier.com or call 888-837-6437.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.






