Experiments
Here are experiments our science specialists have selected to support the IB* topic.
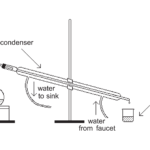
Fractional Distillation
Experiment #8 from Chemistry with Vernier
In this experiment, you will
- Observe what happens when a liquid-liquid mixture is heated and allowed to boil over a period of time.
- Determine percent composition of ethanol and water in the fraction from its density.
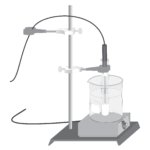
Effect of Temperature on Solubility of a Salt
Experiment #12 from Chemistry with Vernier
In this experiment, you will
- Study the effect of changing temperature on the amount of solute that will dissolve in a given amount of water.
- Plot a solubility curve.
Liquid Chromatography
Experiment #18 from Advanced Chemistry with Vernier
In this experiment, you will
- Conduct an isocratic, liquid-chromatographic separation.
- Conduct a step gradient, liquid-chromatographic separation.
- Complete the necessary measurements and calculations to evaluate the components of a mixture that have been separated by liquid chromatography.
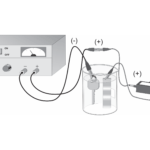
Electroplating
Experiment #21 from Advanced Chemistry with Vernier
In this experiment, you will
- Prepare and operate an electrochemical cell to plate copper onto a brass surface.
- Measure the amount of copper that was deposited in the electroplating process.
- Calculate the amount of energy used to complete the electroplating process.
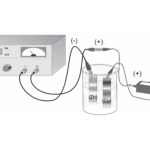
Determining Avogadro's Number
Experiment #31 from Advanced Chemistry with Vernier
In this experiment, you will
- Prepare an electrochemical cell to oxidize a copper electrode.
- Measure the amount of copper that was deposited in the electroplating process and determine the average current used.
- Calculate a value for Avogadro’s number and compare it to the accepted value.
- Educational Standard
- International Baccalaureate (IB)
- Subject
- Chemistry
- Section
- Core
- Topic
- 1. Stoichiometric Relationships
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
