Experiments
Here are experiments our science specialists have selected to support the IB* topic.
The Decomposition of Hydrogen Peroxide
Experiment #12 from Advanced Chemistry with Vernier
In this experiment, you will
- Conduct the catalyzed decomposition of hydrogen peroxide under various conditions.
- Calculate the rate constant for the reaction.
- Determine the rate law expression for the reaction.
- Calculate the activation energy for the reaction.
The Rate and Order of a Chemical Reaction
Experiment #25 from Advanced Chemistry with Vernier
In this experiment, you will
- Conduct the reaction of KI and FeCl3 using various concentrations of reactants.
- Determine the order of the reaction in KI and FeCl3.
- Determine the rate law expression for the reaction.
The Base Hydrolysis of Ethyl Acetate
Experiment #29 from Advanced Chemistry with Vernier
In this experiment, you will
- Conduct the base hydrolysis of ethyl acetate under various conditions.
- Calculate the rate law constant, k, for the reaction.
- Determine the rate law expression for the reaction.
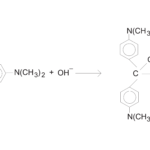
Rate Law Determination of the Crystal Violet Reaction
Experiment #30 from Chemistry with Vernier
In this experiment, you will
- Observe the reaction between crystal violet and sodium hydroxide.
- Monitor the absorbance of the crystal violet solution with time.
- Graph Absorbance vs. time, ln Absorbance vs. time, and 1/Absorbance vs. time.
- Determine the order of the reaction.
- Determine the rate constant, k, and the half-life for this reaction.

Reaction Rates
Experiment #22 from Investigating Chemistry through Inquiry
In the Preliminary Activity, you will use a Gas Pressure Sensor to monitor the pressure increase due to the production of oxygen gas inside an Erlenmeyer flask as potassium iodide catalytically decomposes hydrogen peroxide.
After completing the Preliminary Activity, you will first use reference sources to find out more about reaction rates and factors that influence them before you choose and investigate a researchable question dealing with reaction rates.
- Educational Standard
- International Baccalaureate (IB)
- Subject
- Chemistry
- Section
- Core
- Topic
- 6. Chemical Kinetics
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
