Experiments
Here are experiments our science specialists have selected to support the IB* topic.
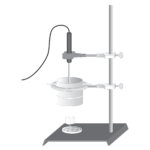
Energy Content of Foods
Experiment #16 from Chemistry with Vernier
In this experiment, you will
- Determine the energy released from various foods as they burn.
- Look for patterns in the amounts of energy released during burning of different foods.
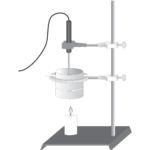
Energy Content of Fuels
Experiment #17 from Chemistry with Vernier
In this experiment, you will
- Compare the heat of combustion for paraffin wax and ethanol.
- Calculate the heat of combustion and percent efficiency for both fuels.
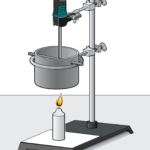
Investigating the Energy Content of Fuels
Experiment #7 from Investigating Chemistry through Inquiry
In the Preliminary Activity, you will determine the heat of combustion of paraffin wax (in kJ/g). You will first use the energy from burning paraffin wax to heat a known quantity of water. By monitoring the temperature of the water, you can find the amount of heat transferred to it (in kJ), using the formula
where q is heat, Cp is the specific heat capacity of water, m is the mass of water, and Δt is the change in temperature of the water. Finally, the amount of fuel burned will be taken into account by calculating the heat per gram of paraffin wax consumed in the combustion.
After completing the Preliminary Activity, you will first use reference sources to find out more about calorimetry and fuels before you choose and investigate a researchable question.
- Educational Standard
- International Baccalaureate (IB)
- Subject
- Chemistry
- Section
- Options
- Topic
- C. Energy
* The IB Diploma Program is an official program of the International Baccalaureate Organization (IBO) which authorizes schools to offer it. The material available here has been developed independently of the IBO and is not endorsed by it.
