Determining the Concentration of a Solution: Beer’s Law
Experiment #17 from Advanced Chemistry with Vernier
Introduction
The primary objective of this experiment is to determine the concentration of an unknown copper (II) sulfate solution. The CuSO4 solution used in this experiment has a blue color, so Colorimeter users will be instructed to use the red LED. Spectrometer users will determine an appropriate wavelength based on the absorbance spectrum of the solution. A higher concentration of the colored solution absorbs more light (and transmits less) than a solution of lower concentration.
You will prepare five copper (II) sulfate solutions of known concentration (standard solutions). Each solution is transferred to a small, rectangular cuvette that is placed into the Colorimeter or Spectrometer. The amount of light that penetrates the solution and strikes the photocell is used to compute the absorbance of each solution. When you graph absorbance vs. concentration for the standard solutions, a direct relationship should result. The direct relationship between absorbance and concentration for a solution is known as Beer’s law.
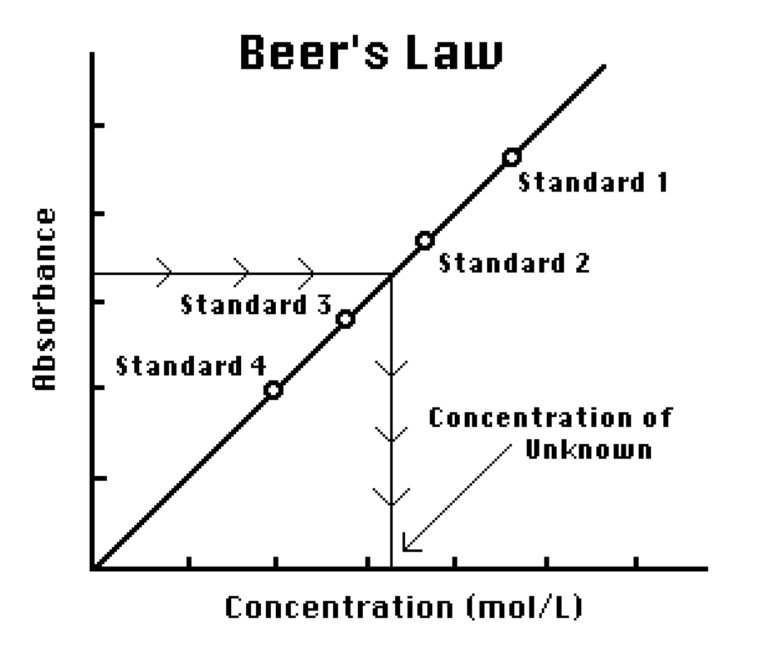
You will determine the concentration of an unknown CuSO4 solution by measuring its absorbance. By locating the absorbance of the unknown on the vertical axis of the graph, the corresponding concentration can be found on the horizontal axis. The concentration of the unknown can also be found using the slope of the Beer’s law curve.
Objectives
In this experiment, you will
- Prepare and test the absorbance of five standard copper (II) sulfate solutions.
- Calculate a standard curve from the test results of the standard solutions.
- Test the absorbance of a copper (II) sulfate solution of unknown molar concentration.
- Calculate the molar concentration of the unknown CuSO4 solution.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 2

Option 3

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #17 of Advanced Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.



