Determining the Free Chlorine Content of Swimming Pool Water
Experiment #33 from Chemistry with Vernier
- Education Level
- High School
- Subject
- Chemistry
Introduction
Physicians in the nineteenth century used chlorine water as a disinfectant. Upon the discovery that certain diseases were transmitted by water, it became common for municipalities to chlorinate public water supplies. It is now standard practice to add chlorine to swimming pools and hot tubs. Chlorine reacts with water to form hypochlorous acid (HOCl) and hypochlorite ion (ClO–).
The chlorine that exists in water as HOCl and OCl– is known as free chlorine. Free chlorine can kill bacteria, prevent algae growth, and oxidize iron to form a precipitate that can be removed from a pool by the filtering system. Swimming pool operators try to maintain a desired range of 1.0 to 1.5 mg/L of free chlorine for proper sanitation.
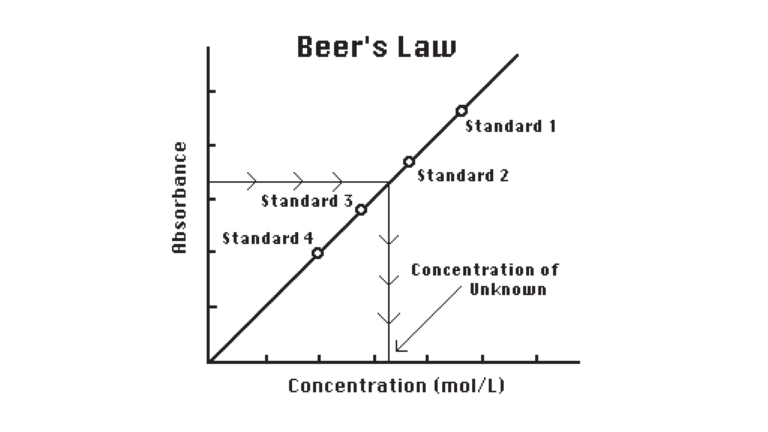
In this experiment, you will use a Colorimeter to determine the amount of free chlorine in a sample of swimming pool or hot tub water. You will first measure the absorbance of green light (565 nm) by aqueous solutions of known chlorine concentration. From the resulting graph of absorbance vs. free chlorine concentration (Beer’s law), you will be able to determine the free chlorine content of your swimming pool sample.
A compound called DPD (N, N-diethyl-p-phenylenediamine) is reacted with the chlorine in each sample. The DPD is oxidized, forming a magenta (red) product. The intensity of the sample’s color is directly proportional to its chlorine concentration.
Objectives
In this experiment, you will
- Prepare free chlorine standard solutions.
- Use a Colorimeter to measure the absorbance of each standard solution.
- Plot a graph of absorbance of free chlorine vs. concentration.
- Use a Colorimeter and your Beer’s law plot to determine the amount of free chlorine in a sample of swimming pool or hot tub water.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 3

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #33 of Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.



