Fossil Fuels
Experiment #26 from Investigating Environmental Science through Inquiry
- Subject
- Environmental Science
Introduction
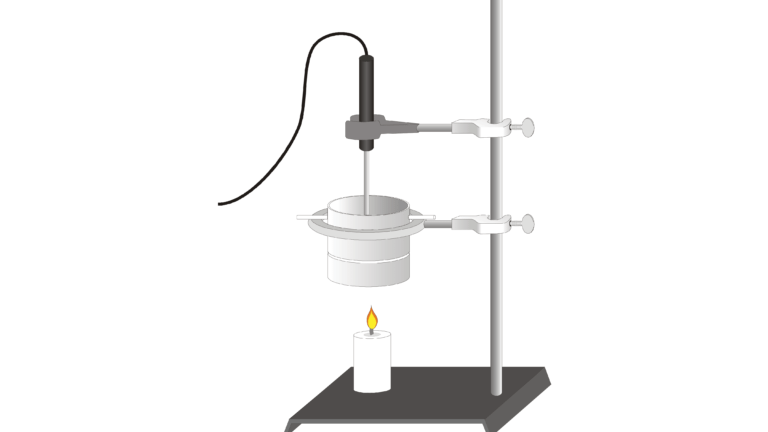
Hydrocarbons are compounds containing only hydrogen and carbon atoms. Many common fuels such as gasoline, diesel fuel, heating oil, aviation fuel, and natural gas are essentially mixtures of hydrocarbons. Paraffin wax, used to make many candles, is a mixture of hydrocarbons with the representative formula C25H52.
Ethyl alcohol, a substituted hydrocarbon with the formula C2H5OH, is used as a gasoline additive (gasohol) and as a gasoline substitute.
Objectives
In the Preliminary Activity, you will determine the heat of combustion of paraffin wax (in kJ/g). You will first use the energy from burning paraffin wax to heat a known quantity of water. By monitoring the temperature of the water, you can find the amount of heat transferred to it (in kJ), using the formula
where q is heat, Cp is the specific heat capacity of water, m is the mass of water, and Δt is the change in temperature of the water. Finally, the amount of fuel burned will be taken into account by calculating the heat per gram of paraffin wax consumed in the combustion.
After completing the Preliminary Activity, you will first use reference sources to find out more about fossil fuel energy before you choose and investigate a researchable question.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Biology
- C4.2.19—Release of carbon dioxide into the atmosphere during combustion of biomass, peat, coal, oil and natural gas
- D4.3.1—Anthropogenic causes of climate change
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #26 of Investigating Environmental Science through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


