Introduction
If you examined the pressure-volume behavior of a gas, you would have performed one of the thermodynamic processes involved in the cycle found in the operation of a heat engine. This process is known as an isothermal expansion – so named because the data were collected slowly enough that the temperature of the gas in the system remained constant.
In this experiment, you will examine some thermodynamic processes to understand how the internal energy of the system (Eint or U) is affected by exchanges of energy between the system and the surroundings.
Objectives
In this experiment, you will
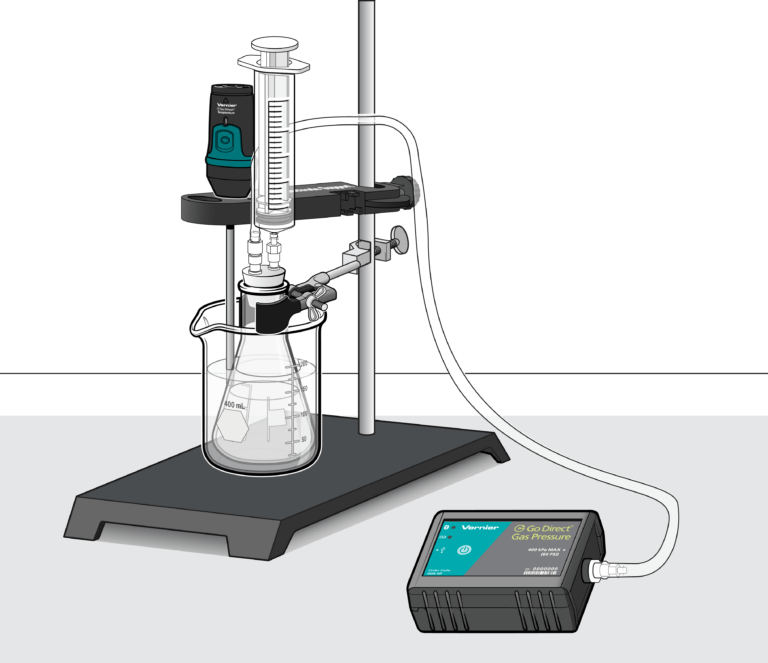
- Design and create a thermodynamic system consisting of a flask, tubing, syringe, and pressure sensor.
- Relate the terms isothermal, isochoric, isobaric and adiabatic to various thermodynamic processes, and how to move your thermodynamic system through these processes.
- Collect pressure, volume and temperature data for three of these processes.
- Analyze the various P–V processes to keep track of the work (W) done by or on the enclosed gas and the heat (Q) transferred between the gas and the surroundings.
- Use the first law of thermodynamics to account for the change in internal energy in each of these processes.
- Determine the total work done by enclosed gas in various thermodynamic cyclic processes.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Correlations
Teaching to an educational standard? This experiment supports the standards below.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #2 of Advanced Physics with Vernier — Beyond Mechanics. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.





