One of the most disappointing things for a teacher is not being able to provide evidence for a claim that students find difficult to accept. When students ask how it is possible to know that CaCl2 produces three ions, while NaNO3 produces only two, it is difficult to provide an easy, convincing answer. Larry Dukerich, a chemistry and physics teacher with over 34 years of teaching experience, developed an extension to Experiment 14, “Conductivity of Solutions,” in Chemistry with Vernier, to help demonstrate the existence of polyatomic ions.
Solid substances known as salts (e.g., potassium chloride, potassium nitrate), yield ions when dissolved in water. These ions permit the flow of an electric current through the solution. Increasing the ion concentration increases the ability with which the solution carries an electric current and results in a higher conductivity. This experiment can provide data to support the claim that some ions are polyatomic.
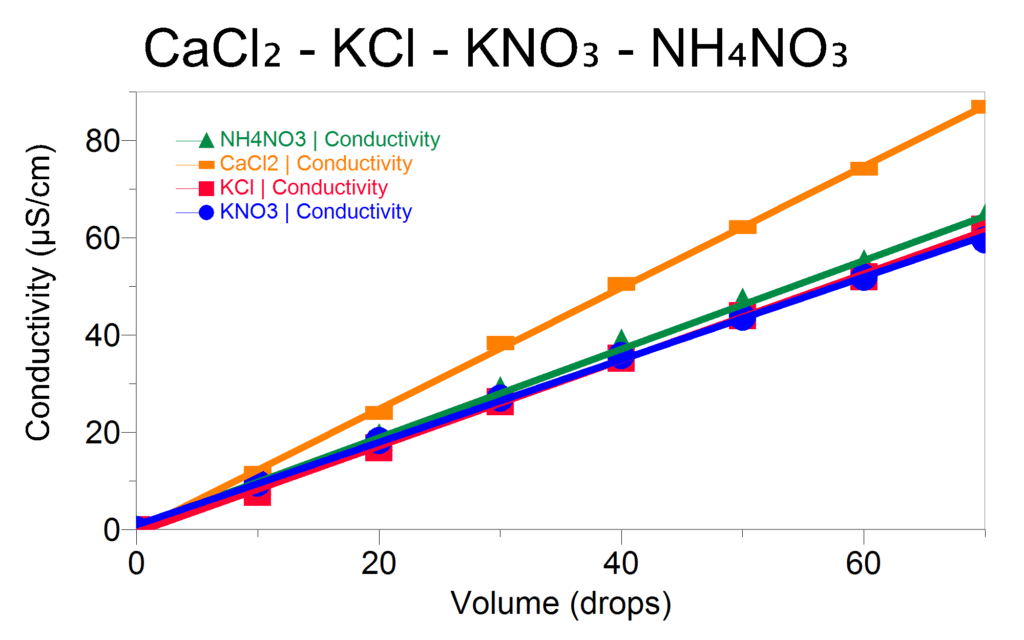
Students and instructors can perform trials with solutions of different salts, including KCl, CaCl2, KNO3, and NH4NO3. Comparing the graphs of conductivity vs. drops of solution added for the electrolytes shows that the slope for CaCl2 is 1.5 times as great as that of KCl. Since conductivity is proportional to the number of ions in solution, the data will support the hypothesis that each formula unit of CaCl2 produces three ions for every two produced by KCl. However, the existence of polyatomic ions still needs to be addressed.
In the procedure, the next set of trials investigates the conductivity of KNO3 and NH4NO3, both of which contain polyatomic ions. The evidence for polyatomic ions is noticeable when comparing the slopes of the graphs of conductivity vs. number of drops for solutions of KNO3 and NH4NO3 to those of KCl and CaCl2. The KNO3 and NH4NO3 graphs will have slopes agreeing with KCl but not CaCl2, suggesting both KNO3 and NH4NO3 dissociate into two ions.
