By Jack Randall
Long ago, when I was a high school chemistry teacher, my students ran a forensics experiment using a Spectronic 20 spectrophotometer. The students measured the absorbance spectra of food dyes in various sports drinks to determine which drink had been “poisoned.” The Spectronic 20 used by my students worked very well, but this was long ago, and it took upwards of 30 minutes to collect a single absorbance spectrum; thus, the entire experiment consumed two to three days of lab time. Now, we have the Vernier SpectroVis Plus spectrophotometer that can collect an absorbance spectrum in seconds, and this interesting, fun experiment can easily be completed in less than an hour.
In the experiment, the students are given several sports drink samples that have been found in a school gym locker room. The drinks have a blue, purple, or green color. One of the drinks has been “poisoned” by the addition of copper (II) sulfate, CuSO4. Because the sports drinks are colored, the bluish color of the CuSO4 cannot be spotted by the naked eye. However, the SpectroVis Plus can see the CuSO4. The students measure the absorbance spectra of the seven FD&C food dyes acceptable for use with foods, a solution of CuSO4, and several sports drink samples. By comparing the graphs, the students can find the “poisoned” sports drink, as well as determine the specific food dyes added to each beverage.
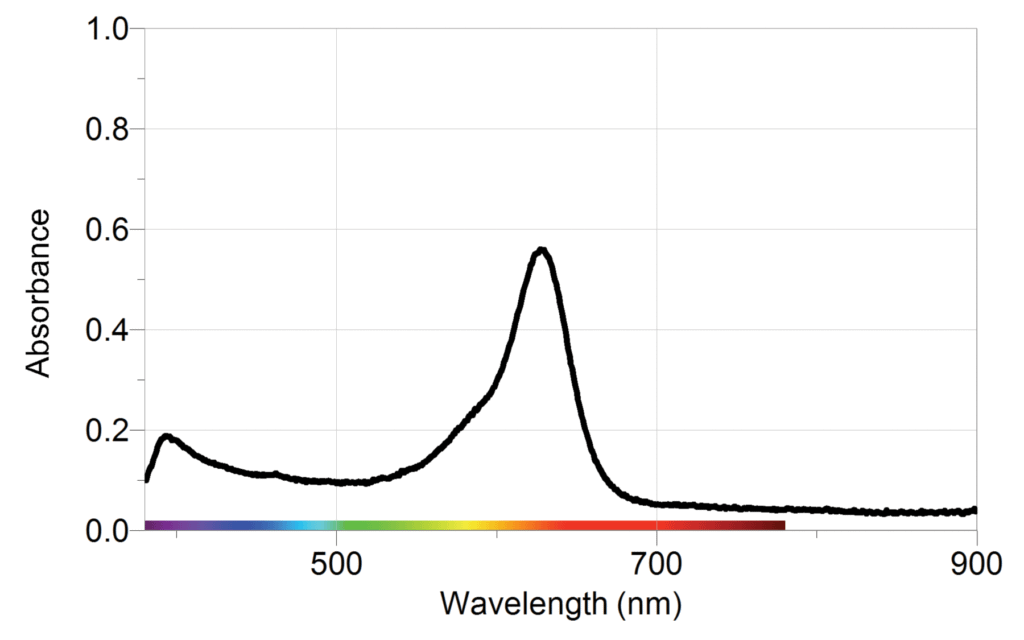
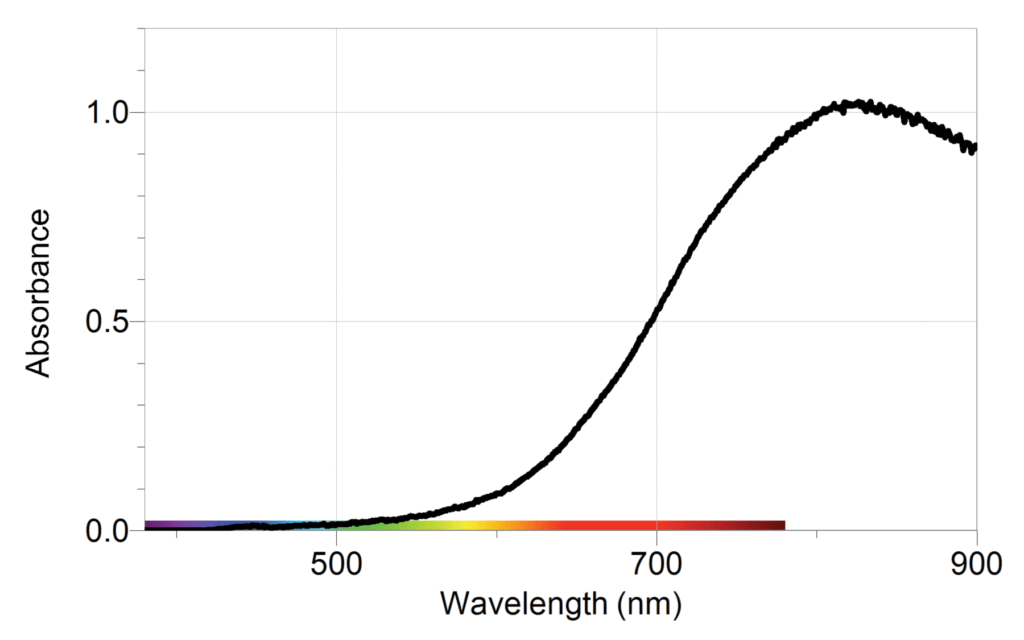
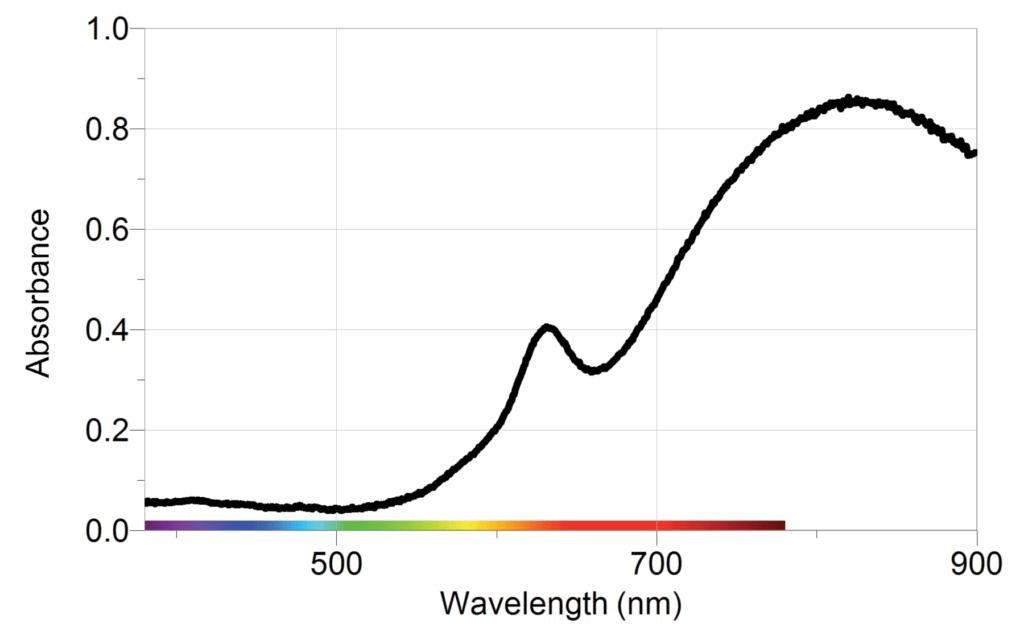
The sample graphs below show typical results from the experiment.