Introduction
In order for cells to survive, they must constantly exchange ions, gases, nutrients, and wastes with their environment. These exchanges take place at the cell’s surface. To perform this function efficiently, there must be an adequate ratio between the cell’s volume and its surface area. As a cell’s volume increases, its surface area increases, but at a decreased rate. If you continued to increase the cell’s volume, it would soon be unable to efficiently exchange materials and the cell would die. This is the reason that the kidney cell of an elephant is the same general size as a mouse kidney cell.
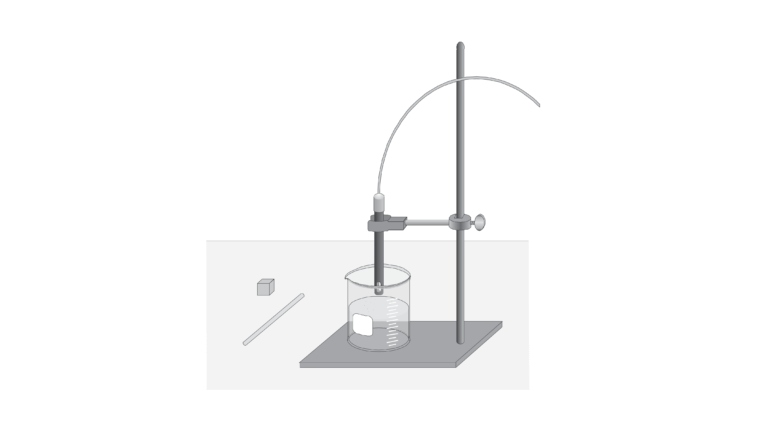
In this lab activity, you will use agar cubes, which have a high salt content, as cell models. You will investigate how increasing a cell’s surface area while maintaining an equal volume affects the rate of material exchange with the environment. When the agar cubes are placed in distilled water, they will begin to dissolve, releasing sodium and chloride ions. The solution’s conductivity, measured by a Conductivity Probe, is proportional to the ion concentration in the solution.
Objectives
In this experiment, you will
- Use agar cubes cut into various size blocks to simulate cells.
- Use a Conductivity Probe to measure the quantity of ions in a solution.
- Determine the relationship between the surface area and volume of a cell.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Correlations
Teaching to an educational standard? This experiment supports the standards below.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #2 of Biology with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

