Lead Storage Batteries
Experiment #29 from Chemistry with Vernier
- Education Level
- High School
- Subject
- Chemistry
Introduction
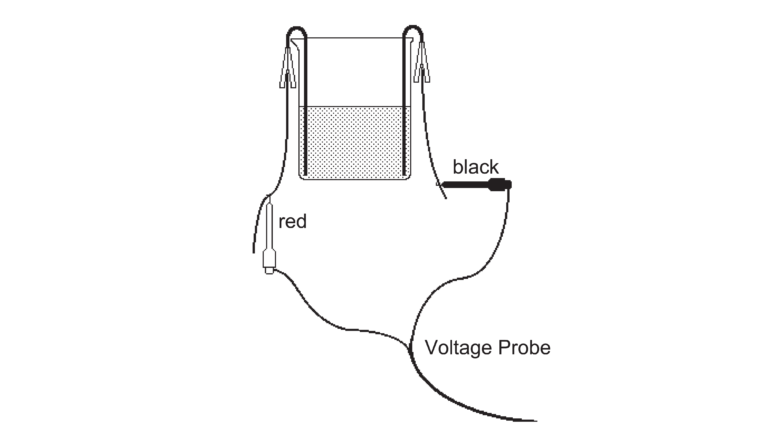
Two or more wet or dry cells connected in series make a battery. A car battery is generally a lead storage battery containing lead and lead oxide plates in sulfuric acid solution. In this experiment, you will construct a lead storage cell and use a direct-current power supply to charge it. You will use a Voltage Probe to measure the cell’s voltage, and then use the cell to power an electric motor.
Objectives
In this experiment, you will
- Construct a lead storage cell.
- Use a Voltage Probe to measure a cell’s voltage.
- Use the cell to power an electric motor.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Reactivity 3.2.7—Secondary (rechargeable) cells involve redox reactions that can be reversed using electrical energy.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #29 of Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

