Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM
In my last blog post, I broke down Question 1 from the 2024 AP Chemistry Exam, which focused on where students struggled with titrations and calorimetry. Now, let’s look at Free-Response Question 3—another tough one for students. This question asked them to apply their knowledge of oxidation-reduction reactions, periodic trends, and the properties of alloys.As someone who’s taught electrochemistry more times than I can count, I’ve seen how tricky these topics can be without strong foundational understanding and opportunities to see the reactions in action. Below, I’ll highlight the most common knowledge gaps identified in the Chief Reader Report, followed by some Vernier experiments that help reinforce these ideas through hands-on lab work and real-time data collection.
Free Response Question 3: Sterling Silver & Electroplating
Topics Covered: Structure of Metals and Alloys, Periodic Trends, Stoichiometry, Oxidation/Reduction Reactions, Cell Potential, Electrolysis, Faraday’s Law*
Max Score: 10
Mean Student Score: 4.42
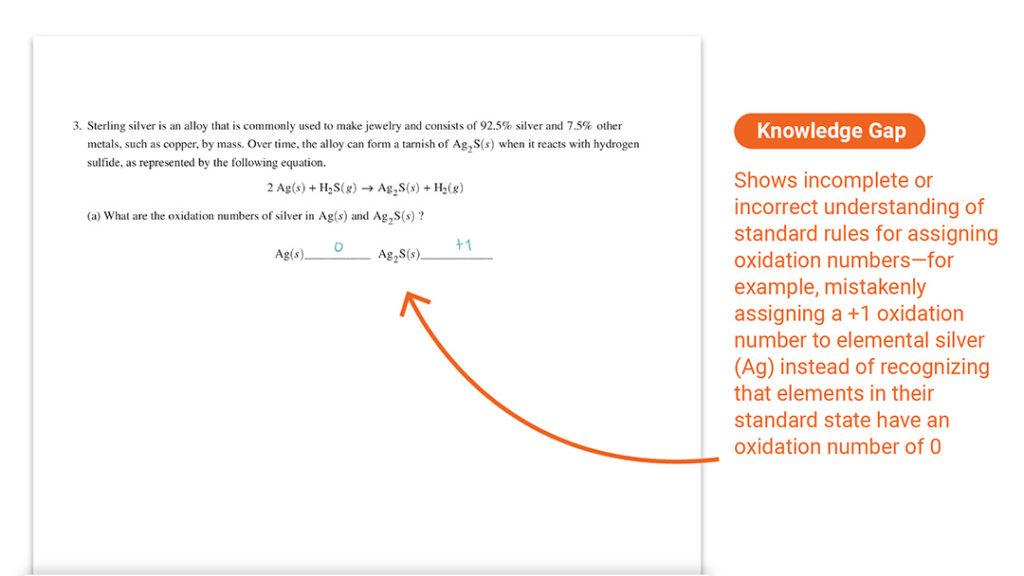
Question 3 Part (a): Assigning Oxidation Numbers

Question 3 Part (b)(i): Classifying Sterling Silver as a Substitutional Alloy

Question 3 Part (b)(ii): Understanding Atomic Radius & Periodic Trends

Question 3 Part (c): Calculating the Moles of Removed Atoms
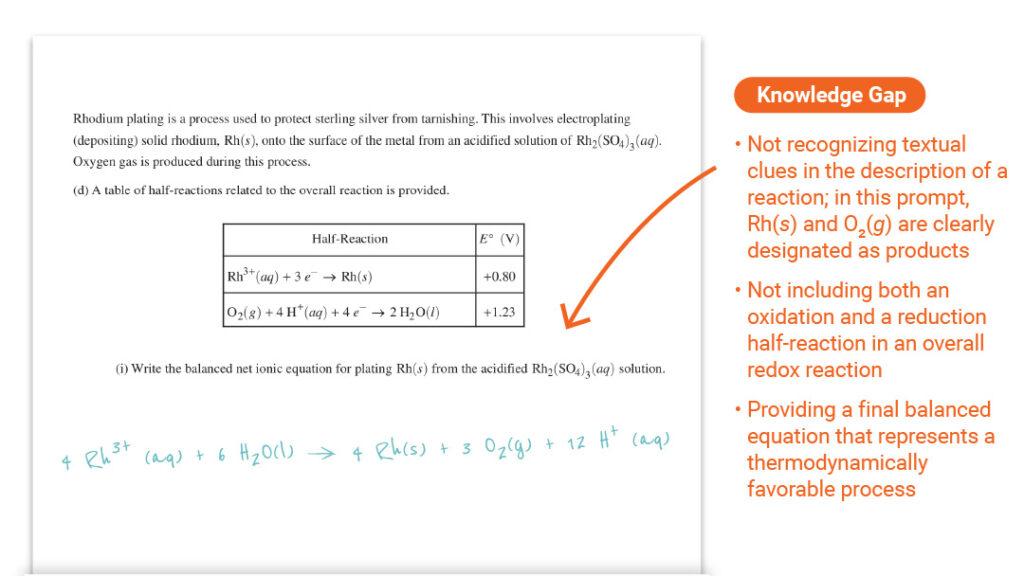
Question 3 Part (d)(i): Writing Ionic Equations
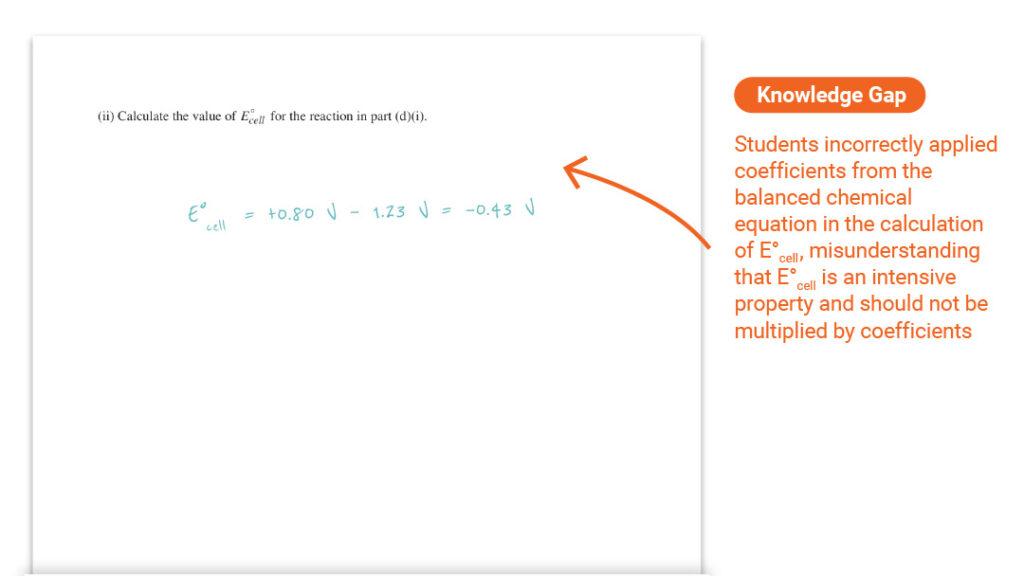
Question 3 Part (d)(ii): Calculating the Value of E°cell
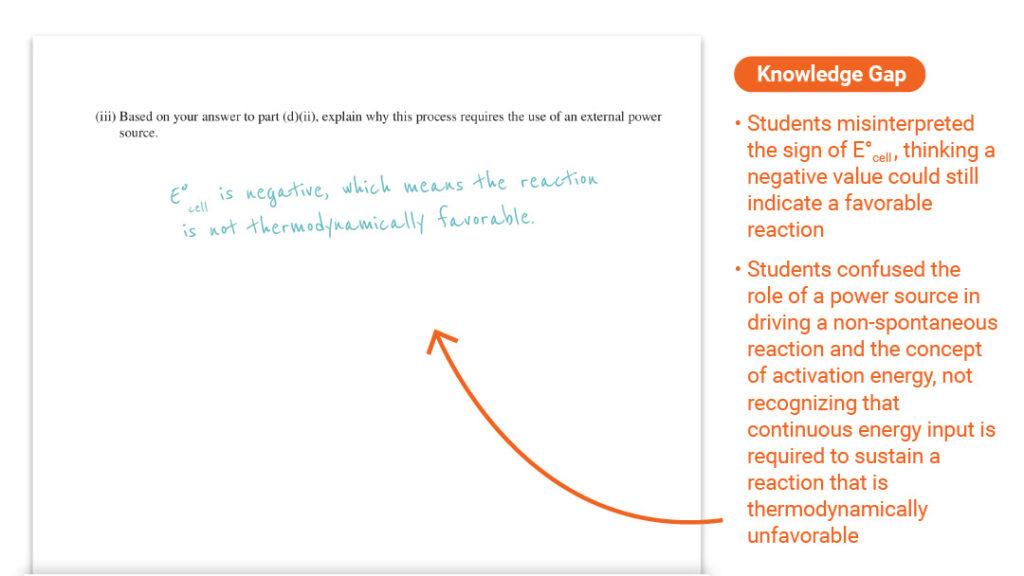
Question 3 Part (d)(iii): Constructing Explanations from Evidence
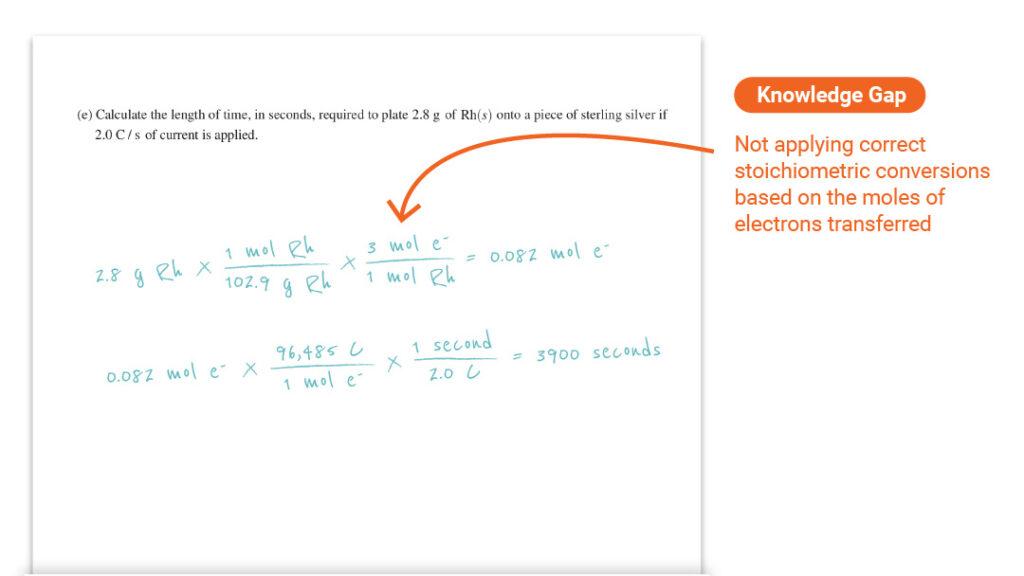
Question 3 Part (e): Calculating Reaction Time
Question 3: Key Takeaways
Once again, the Chief Reader Report has some solid advice for helping students get over the hurdles in Question 3. And as someone who’s taught electrochemistry more times than I can count, I completely agree—these are the areas where students benefit from more clarity and practice.
- Precision in Scientific Language: Students need more practice articulating key concepts using specific vocabulary, such as “principal energy levels” and “thermodynamic favorability.”
- Equation Writing Skills: Students should practice extracting textual clues to correctly write and balance chemical equations.
- Sign Conventions in Thermodynamics: Algebraic errors in electrochemistry are still a big issue. Reinforce sign conventions and make sure students understand what the signs actually mean when defining thermodynamic favorability.
Try These Experiments in Your Lab
In this experiment, students write balanced half-reactions, measure and interpret cell potentials (using a voltage probe and an Electrochemistry Half-Cell Plate), and rank metals by reduction potential. Throughout the investigation, they consider green chemistry principles—an ideal setup for reinforcing the electrochemistry and redox reasoning required in Question 3. Download it here.
Students use a Go Direct® Constant Current System to observe and measure electroplating, write redox half-reactions, and use the total charge transferred to calculate Avogadro’s number. This experiment offers a hands-on way to connect quantitative electrochemistry with sustainability. Download it here.
In this experiment, students set up an electrochemical cell to electroplate copper onto brass. Using a current sensor, they measure the mass of copper deposited, write oxidation and reduction half-reactions, and apply Faraday’s law to calculate energy usage—directly supporting the quantitative reasoning skills emphasized in electrolysis questions.
This investigation challenges students to explore alloy composition. After dissolving a brass sample in nitric acid, they use a spectrophotometer to analyze the resulting solution to calculate copper content—supporting key concepts from Question 3 related to the structure and makeup of alloys like sterling silver.
Looking for More AP Chemistry Support?
Whether you’re starting a new AP program or looking for new ways to reinforce tricky AP topics, Vernier has you covered. Check out these resources for more webinars, teacher tips, and classroom-ready investigations aligned with the AP Chemistry framework.
- AP Chemistry Exam 2024 Question 1 Breakdown: Read our previous breakdown covering titration and calorimetry.
- Vernier Chemistry Investigations for Use with AP Chemistry: Our AP-aligned lab book includes 16 guided-inquiry and hands-on experiments that help students build conceptual understanding through data collection and analysis.
- AP Chemistry Purchasing Guide: This helpful overview covers recommended sensors and equipment for a complete AP Chemistry lab setup.
- Top Three Topics That Students Miss in AP Chemistry Prep—and How to Tackle Them: This blog post focuses on common student struggles and practical ways to support them with targeted experiments.
- A Mole Lot of Fun: Chemistry Investigations from Avogadro’s Number to Electrodes: This webinar demonstrates two key electrochemistry experiments that connect the mole concept to observable real-world phenomena and follow the principles of green chemistry.
- Recharge Your Lesson Plan! Moving Beyond the Lemon Battery to Teach Electrochemistry: This webinar explores how to use simple materials (string, metal wire, and salt solutions) to create half-cells and investigate the structure of a voltaic cell, electrolytes, and salt bridges.
- Past AP Chemistry Exam Questions: Download free-response questions from past exams along with scoring guidelines, sample responses from exam takers, and scoring distributions.
*All exam questions, knowledge gap summaries, and takeaway commentary are based on the 2024 AP Chemistry Chief Reader Report and supporting materials provided by the College Board.
**AP and Advanced Placement Program are registered trademarks of the College Entrance Examination Board, which was not involved in the production of and does not endorse this product.
We’re here to help! Reach out to chemistry@vernier.com, call 888-837-6437, or drop us a line in the live chat.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.