
Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM

As the AP Exam* season approaches, now is a great time to revisit relevant topics that may have been overlooked during your exam preparation. Certain topics, especially those in electrochemistry, thermodynamics, and kinetics, tend to get missed either because they appear late in the curriculum, leaving little time for thorough coverage, or because their complexity can be daunting, leading some educators to skirt around them. In this blog post, we’re sharing some practical experiments from our lab books that can help you address potential gaps.
1. Electrochemistry: Understanding Voltaic (Galvanic) Cells
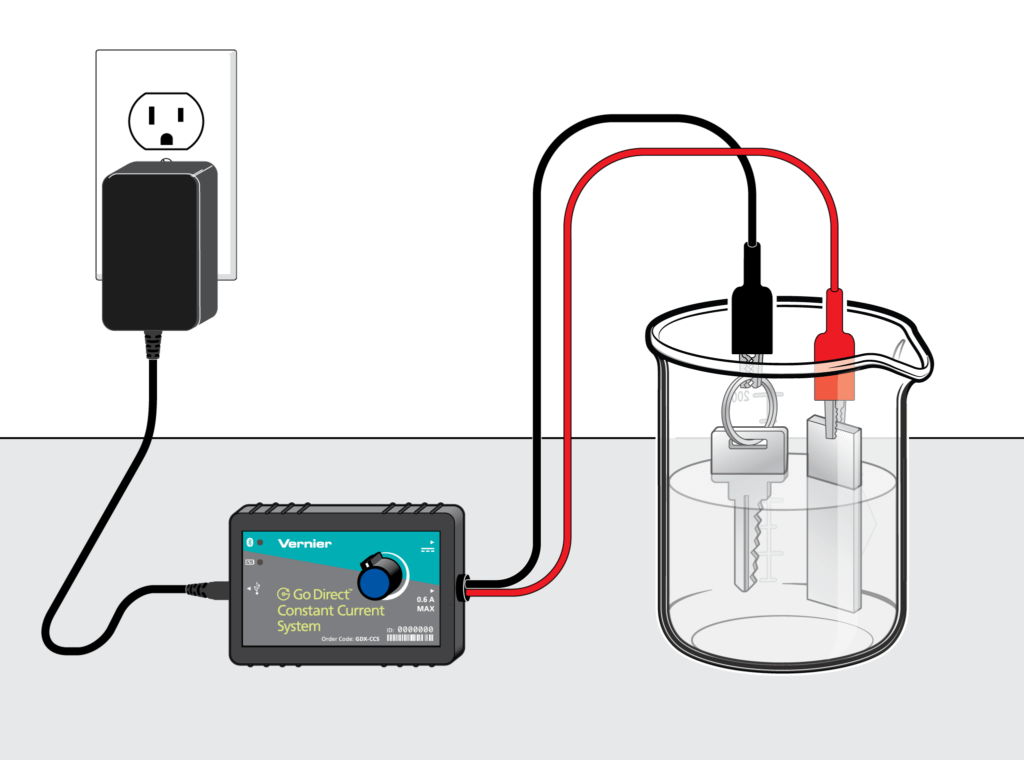
Concepts about electrochemistry frequently surface on the AP Chemistry Exam in questions about chemical reactions. Students need to be able to identify what is being oxidized and what is being reduced, as well identify and understand what is happening at the cathode and anode.
A lot of students memorize mnemonics about this, like “Red Cat” and “An Ox,” but it is essential for students to actually grasp the underlying principles behind it. The following experiments are a great way to get students comfortable with these concepts, including using the Nernst equation:
Recommended Experiments
- Electrochemistry: Voltaic Cells (Experiment #20, Advanced Chemistry with Vernier)
- Electroplating (Experiment #21, Advanced Chemistry with Vernier)
- Determining Avogadro’s Number (Experiment #31, Advanced Chemistry with Vernier)
2. Thermodynamics: Determining Enthalpy
Thermodynamics plays a vital role in understanding the role of energy in chemical reactions. For the AP Exam, students need to understand how to calculate the energies of bonds broken and formed, as well as analyze calorimetry data to determine reaction enthalpy.
We often see students struggle with these concepts, and in particular, with determining the number of bonds broken and made when calculating the enthalpy of a reaction. We recommend trying out these experiments to help build confidence in understanding enthalpy:
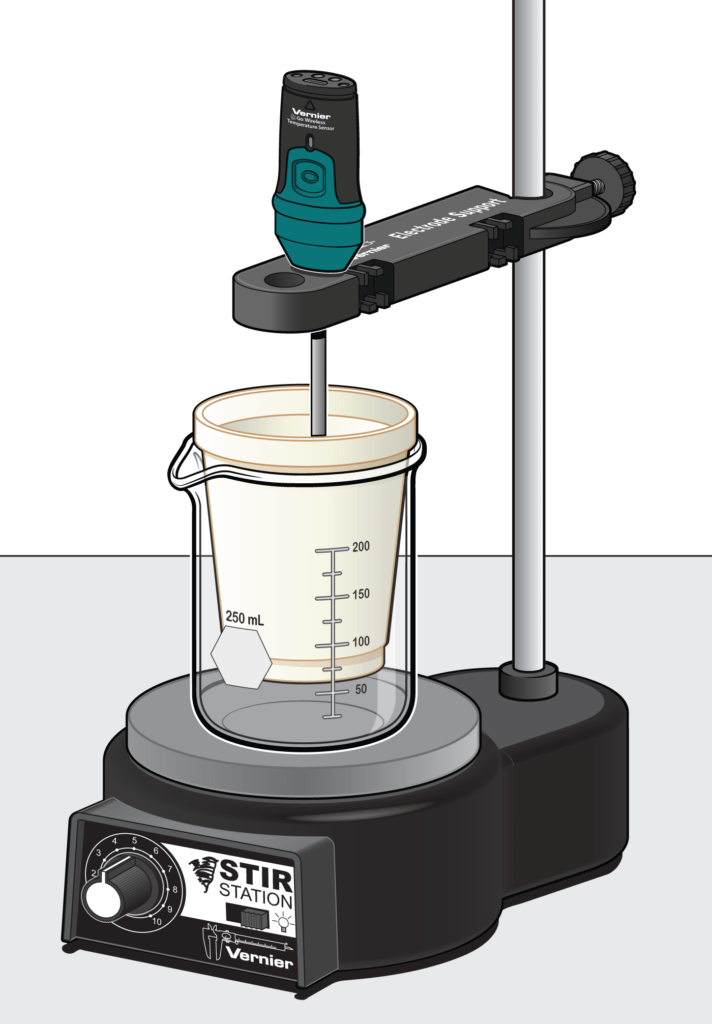
Recommended Experiments
- Determining the Enthalpy of a Chemical Reaction (Experiment #13, Advanced Chemistry with Vernier)
- The Enthalpy of Neutralization of Phosphoric Acid (Experiment #26, Advanced Chemistry with Vernier)
- Burning Accelerants (Experiment #6, Forensic Chemistry Experiments)
- Food Is Fuel (Experiment #01, Food Chemistry Experiments)
3. Kinetics: Understanding Rate Constants
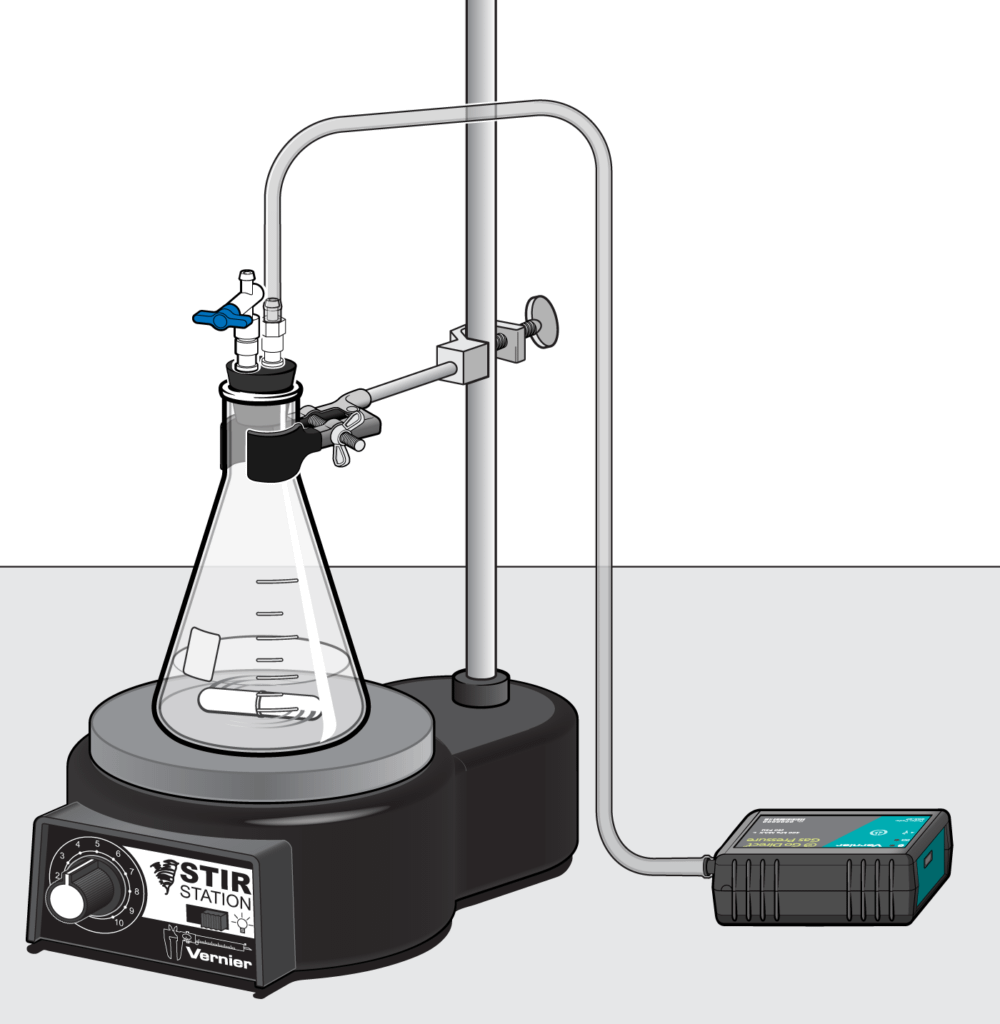
In kinetics, students investigate the rates at which chemical changes occur and what factors might influence those rates. For the AP Exam, students need to navigate between experimental data, rate laws, and a proposed mechanism.
One of the big concepts that students struggle with in this area is understanding graphs of reactant concentration versus time and drawing conclusions about rate constants. Here are some excellent experiments to get students more comfortable with analyzing graphs, tables, and determining the order of reaction:
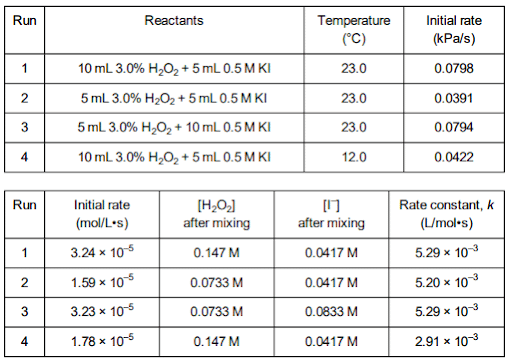
Recommended Experiments
- The Decomposition of Hydrogen Peroxide (Experiment #12, Advanced Chemistry with Vernier)
- The Rate and Order of a Chemical Reaction (Experiment #25, Advanced Chemistry with Vernier)
- Color Countdown Timer (Colorimeter) (Experiment #7B, Forensic Chemistry Experiments)
- Rate Determination and Activation Energy (Experiment #35, Advanced Chemistry with Vernier)
What Vernier experiments have helped you prepare your students for the AP Chemistry Exam? We’d love to hear about your experience! Reach out to us at chemistry@vernier.com or call 888-837-6437.
*AP and Advanced Placement Program are registered trademarks of the College Entrance Examination Board, which was not involved in the production of and does not endorse this product.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.






