
Sharing ideas and inspiration for engagement, inclusion, and excellence in STEM
As we officially move into autumn and begin creeping closer to the spookiest season of the year, it’s the perfect time to add a little bit of pumpkin spice to your science curriculum.
We’re sharing two of our favorite seasonal and spooky data-collection experiments to explore with your students this Halloween season!
Chemistry
Puking Pumpkins!
This experiment is a delightfully grotesque way to engage your students in discussions of chemical kinetics and catalytic decomposition, as well exothermic reactions! You can do this as a demo for your class, or have students bring in carved pumpkins for individual or group experiments. Goggles and gloves should be worn during the experiment.
The Experiment
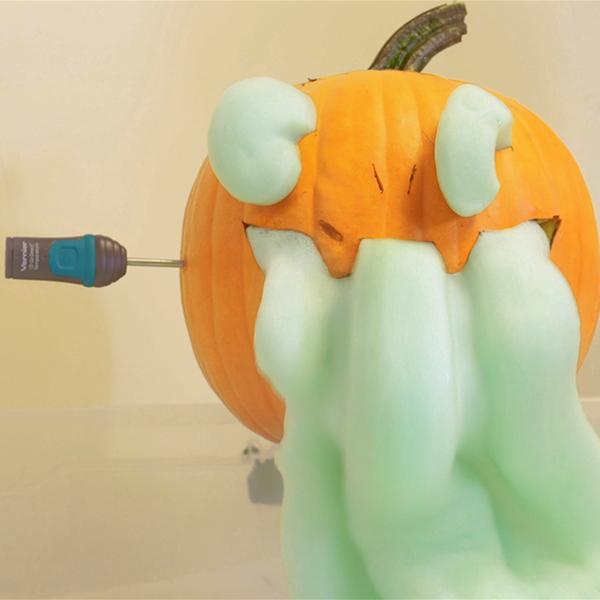
In this activity, we place a small cup of hydrogen peroxide (H2O2), mixed with a few drops of food coloring and dish soap, inside a carved jack-o-lantern. Then, carefully pour saturated potassium iodide (KI) into the solution and quickly put the “lid” back on the pumpkin. Watch as the jack-o’-lantern dramatically regurgitates the decomposed products, “puking” soapy foam through its toothy grin or grimace!
What’s Happening
Hydrogen peroxide decomposes over time into oxygen gas and water. In this experiment, students will speed up the decomposition process by introducing a catalyst to the solution: saturated KI.
Adding food coloring and dish soap will make the effect more visibly exaggerated, as the decomposed oxygen gas fills up and expands the soap bubbles—giving the jack-o’-lantern even more material to spew. And, with a Go Direct® Temperature Probe, students can also track the heat changes, opening up a discussion of exothermic reactions.
Prompt students to share their expectations about what will happen during the experiment. When the hydrogen peroxide decomposes to both liquid (water) and gas (oxygen), do they expect the temperature to change? Why or why not? Why do they think we are adding dish soap to the solution?
What You’ll Need
- A carved jack-o’-lantern (with a “lid”)
- 60 ml of 30% hydrogen peroxide (H2O2) in a beaker or cup
- 20 ml of saturated potassium iodide (KI)
- Food coloring
- Dish soap
- Go Direct Temperature Probe
- Vernier Graphical Analysis® or a LabQuest
Related Experiments
This activity is a great supplement for other topics in your chemistry curriculum. It can pair well with these experiments from our chemistry lab books:
Physics
The Great Pumpkin Drop!
This activity is a dramatic way to explore the acceleration of gravity near the Earth’s surface with your physics students. By analyzing videos of falling pumpkins of varying masses, they will be able to compare different data sets as they fall and open up a discussion of the relationship between mass and gravitational acceleration.
The Experiment
Locate a tall enough structure where you can safely drop a few pumpkins on to a flat surface. Set up an object of known length and good visibility to serve as a scale for the video and make sure your pumpkins and carved jack-o’-lanterns have a clear, contrasting color point that can be tracked in video analysis. Record video of each pumpkin as it drops, and examine the data in video analysis with your students.
To make sure your students obtain useful data for analysis, set up the camera or phone far from the drop and use zoom to get close to the action. Make sure the camera or phone is stable and do not track the pumpkin as it falls. More tips for collecting effective videos can be found here and here.
What’s Happening
It can be difficult to tell that a falling object is accelerating, based on how our eyes and brain work together to show us moving objects. So doing this activity as a video analysis exercise demonstrates that, despite what our brain shows us, falling objects really do start slowly and move faster and faster the farther they fall.

Prompt your students to make predictions about the data you’re collecting. Will the uncarved pumpkins fall faster than the carved ones? Will they fall consistently or at an increasing rate?
It’s valuable for students to measure the acceleration of gravity in multiple ways to underscore that the value of g = 9.8m/s2 consistently crops up—not just in one experiment—and therefore is meaningful regarding the motion of objects near Earth’s surface. The pumpkin drop activity uses the Vernier Video Analysis® method.
What You’ll Need
- Several pumpkins of varying size (may be carved or not, as desired)
- High-visibility scale object
- Camera, tablet, or smartphone to record video
- Vernier Video Analysis
- Optional: tarp or plastic sheeting for easy clean-up
Related Experiments
To explore additional experiments for demonstrating gravity, check out our webinar Measuring g Three Ways, which also demonstrates motion detector and a tossed basketball, and the picket fence falling through a photogate. We also recommend Galileo’s method of using a cart on a ramp at different angles and then extrapolating to the case of the ramp being vertical.
The pumpkin drop is a great supplement for other related topics in your physics curriculum. It can pair well with these experiments from Vernier Video Analysis: Motion and Sports:
- Accelerated Motion (before the pumpkin drop)
- Terminal Velocity (as a follow up to the pumpkin drop)
We’re always here to help! If you have any questions about these experiments or the corresponding Vernier sensors and software, please reach out to chemistry@vernier.com, physics@vernier.com, or call 888-837-6437.
Share this Article

Sign up for our newsletter
Stay in the loop! Beyond Measure delivers monthly updates on the latest news, ideas, and STEM resources from Vernier.






