Investigating Voltaic Cells
Experiment #20 from Investigating Chemistry through Inquiry
- Subject
- Chemistry
Introduction
In electrochemistry, a voltaic cell is a specially prepared system in which an oxidation-reduction reaction occurs spontaneously. This spontaneous reaction produces an easily measured electrical potential. Voltaic cells have a variety of uses.
Objectives
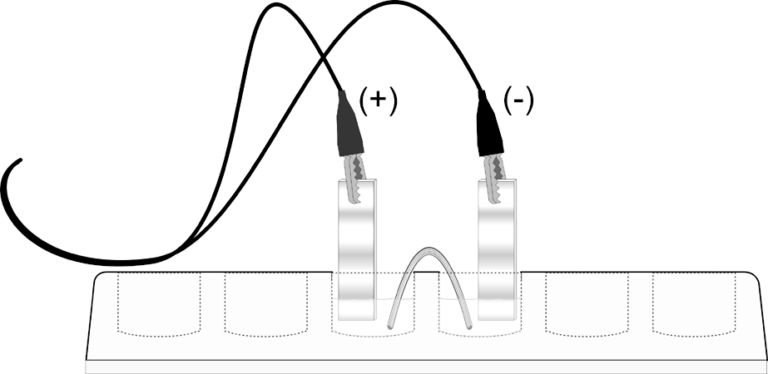
In the Preliminary Activity, you will prepare a semi-microscale voltaic cell in a 24-well test plate. A voltaic cell is constructed by using two metal electrodes and solutions of their respective salts (the electrolyte component of the cell) with known molar concentrations. You will use a Voltage Probe to measure the potential of a voltaic cell with zinc and copper electrodes.
After completing the Preliminary Activity, you will first use reference sources to find out more about electrochemistry and voltaic cells before you choose and investigate a researchable question dealing with voltaic cells.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #20 of Investigating Chemistry through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

